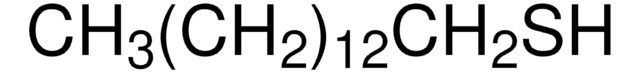O1858
1-Octadecanethiol
98%
Synonym(s):
Mercaptan C18, Octadecyl mercaptan, Stearyl mercaptan
Sign Into View Organizational & Contract Pricing
All Photos(2)
About This Item
Linear Formula:
CH3(CH2)17SH
CAS Number:
Molecular Weight:
286.56
Beilstein:
1811934
EC Number:
MDL number:
UNSPSC Code:
12352103
PubChem Substance ID:
NACRES:
NA.23
Recommended Products
Quality Level
Assay
98%
bp
204-210 °C/11 mmHg (lit.)
mp
30-33 °C (lit.)
density
0.847 g/mL at 25 °C (lit.)
SMILES string
CCCCCCCCCCCCCCCCCCS
InChI
1S/C18H38S/c1-2-3-4-5-6-7-8-9-10-11-12-13-14-15-16-17-18-19/h19H,2-18H2,1H3
InChI key
QJAOYSPHSNGHNC-UHFFFAOYSA-N
Looking for similar products? Visit Product Comparison Guide
Related Categories
General description
1-Octadecanethiol (ODT) is an alkanethiol with a long carbon chain mainly used as a self-assembled monolayer (SAM) for surface modification of a wide range of materials. It facilitates superhydrophobicity with high wettability and low surface energy.
Application
ODT is used in the surface modification of gold nanoparticle (AuNPs)/Nickel (Ni) foam conjugate for the preparation of a 3D surface enhanced Raman spectroscopy(SERS) substrate for the detection of organic pollutants. It may also be used enhance the superhydrophobic properties for different materials like nano-sized copper films, graphene and silica for different applications like mercury detection and electrochemical analysis.
Signal Word
Warning
Hazard Statements
Precautionary Statements
Hazard Classifications
Aquatic Acute 1 - Skin Irrit. 2
Storage Class Code
11 - Combustible Solids
WGK
WGK 3
Flash Point(F)
Not applicable
Flash Point(C)
Not applicable
Personal Protective Equipment
dust mask type N95 (US), Eyeshields, Gloves
Choose from one of the most recent versions:
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Customers Also Viewed
Three-dimensional superhydrophobic surface-enhanced Raman spectroscopy substrate for sensitive detection of pollutants in real environments.
Zhao H, et al.
Journal of Material Chemistry A, 3(8), 4330-4337 (2015)
Fabrication of super-hydrophobic nano-sized copper films by electroless plating.
Liu X, et al.
Thin Solid Films, 518(14), 3731-3734 (2010)
Electrochemical investigation of dynamic interfacial processes at 1-octadecanethiol-modified copper electrodes in halide-containing solutions.
Ma HY, et al.
Electrochimica Acta, 48(28), 4277-4289 (2003)
David Wakerley et al.
Nature materials, 18(11), 1222-1227 (2019-08-07)
The aqueous electrocatalytic reduction of CO2 into alcohol and hydrocarbon fuels presents a sustainable route towards energy-rich chemical feedstocks. Cu is the only material able to catalyse the substantial formation of multicarbon products (C2/C3), but competing proton reduction to hydrogen
Ken Kobayashi et al.
Journal of chromatography. A, 1110(1-2), 95-101 (2006-02-14)
Three types of thiol compounds (n-octadecanethiol, thiophenol, and 2-phenylethanethiol) were used to modify the gold-coated polystyrene particles (dp. 5microm) to prepare a stationary phase for capillary liquid chromatography through the formation of self-assembled monolayer. The column with n-octadecanethiol-modified gold-coated polystyrene
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service