93690
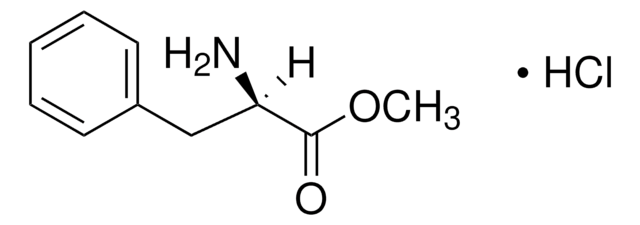
L-Tryptophan ethyl ester hydrochloride
≥99.0% (AT)
Sign Into View Organizational & Contract Pricing
All Photos(1)
About This Item
Empirical Formula (Hill Notation):
C13H16N2O2 · HCl
CAS Number:
Molecular Weight:
268.74
Beilstein:
3919010
EC Number:
MDL number:
UNSPSC Code:
12352209
PubChem Substance ID:
NACRES:
NA.22
Recommended Products
Quality Level
Assay
≥99.0% (AT)
form
powder
optical activity
[α]20/D +10±1°, c = 2% in H2O
reaction suitability
reaction type: solution phase peptide synthesis
mp
220-225 °C (dec.)
application(s)
peptide synthesis
SMILES string
Cl.CCOC(=O)[C@@H](N)Cc1c[nH]c2ccccc12
InChI
1S/C13H16N2O2.ClH/c1-2-17-13(16)11(14)7-9-8-15-12-6-4-3-5-10(9)12;/h3-6,8,11,15H,2,7,14H2,1H3;1H/t11-;/m0./s1
InChI key
PESYCVVSLYSXAK-MERQFXBCSA-N
Looking for similar products? Visit Product Comparison Guide
Storage Class Code
11 - Combustible Solids
WGK
WGK 3
Flash Point(F)
Not applicable
Flash Point(C)
Not applicable
Personal Protective Equipment
dust mask type N95 (US), Eyeshields, Gloves
Choose from one of the most recent versions:
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Kento Takayama et al.
Journal of natural medicines, 75(1), 116-128 (2020-10-21)
Indole is produced from dietary tryptophan by tryptophanase in intestinal bacteria, such as Escherichia coli. In the liver, indole is converted into indoxyl sulfate, a uremic toxin and risk factor for chronic kidney disease (CKD). Probiotics and prebiotics are currently
Anikó Takátsy et al.
Journal of molecular recognition : JMR, 19(4), 270-274 (2006-05-17)
Studies of molecular recognition of chiral compounds by proteins are of importance from many points of view. The biological role of proteins in their interaction with small molecules is of fundamental interest and can be used in many different fields
F L Tse et al.
The Journal of pharmacy and pharmacology, 36(9), 633-636 (1984-09-01)
Sandoz compound 57-118 is a mixture of tryptophan ethyl ester amide derivatives (analogues I-V) possessing one of five fatty acid chains which differ in chain length, configuration, or the degree of unsaturation. The relative absorption of each of the five
V Iu Shviadas et al.
Biokhimiia (Moscow, Russia), 45(4), 629-635 (1980-04-01)
The hydrolysis of L-tryptophane ethyl ester catalyzed by alpha-chymotrypsin and the effect of ethyl ster of D-tryptophane on the course of the reaction were studied. A kinetic pattern of a three-step enzymatic reaction based on the assumption that the enzyme
Diana E Schlamadinger et al.
The journal of physical chemistry. B, 113(44), 14769-14778 (2009-10-13)
Ultraviolet resonance Raman (UVRR) spectra of tryptophan compounds in various solvents and a model peptide are presented and reveal systematic changes that reflect solvent polarity, hydrogen bond strength, and cation-pi interaction. The commonly utilized UVRR spectral marker for environment polarity
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service