All Photos(2)
About This Item
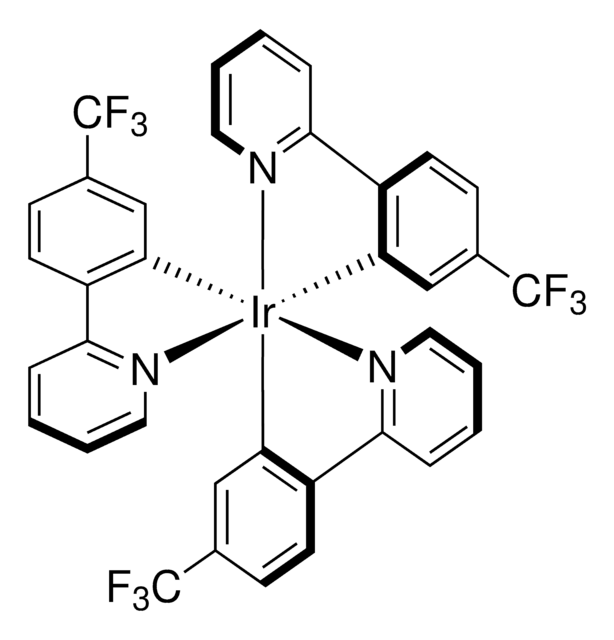
Empirical Formula (Hill Notation):
C45H48IrN3
CAS Number:
Molecular Weight:
823.10
UNSPSC Code:
12352101
NACRES:
NA.22
Recommended Products
form
powder
reaction suitability
reaction type: Photocatalysis
reagent type: catalyst
Related Categories
Application
This photocatalyst facilitates a variety of transformations using visible light, including the benzylation of aldehydes with alcohols.
Product can be used with our line of photoreactors: Including Penn PhD (Z744035) & SynLED 2.0 (Z744080)
Product can be used with our line of photoreactors: Including Penn PhD (Z744035) & SynLED 2.0 (Z744080)
Other Notes
Spin-Center Shift-Enabled Direct Enantioselective α-Benzylation of Aldehydes with Alcohols
Photocatalytic Generation of 2-Azolyl Radicals: Intermediates for the Azolylation of Arenes and Heteroarenes via C–H Functionalization
Visible-Light Photocatalytic Decarboxylation of α,β-Unsaturated Carboxylic Acids: Facile Access to Stereoselective Difluoromethylated Styrenes in Batch and Flow
Photocatalytic Generation of 2-Azolyl Radicals: Intermediates for the Azolylation of Arenes and Heteroarenes via C–H Functionalization
Visible-Light Photocatalytic Decarboxylation of α,β-Unsaturated Carboxylic Acids: Facile Access to Stereoselective Difluoromethylated Styrenes in Batch and Flow
Storage Class Code
11 - Combustible Solids
WGK
WGK 3
Flash Point(F)
Not applicable
Flash Point(C)
Not applicable
Certificates of Analysis (COA)
Search for Certificates of Analysis (COA) by entering the products Lot/Batch Number. Lot and Batch Numbers can be found on a product’s label following the words ‘Lot’ or ‘Batch’.
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Xiao-Jing Wei et al.
ACS catalysis, 7(10), 7136-7140 (2017-11-08)
The development of synthetic methodologies which provide access to both stereoisomers of α,β-disubstituted olefins is a challenging undertaking. Herein, we describe the development of an operationally simple and stereoselective synthesis of difluoromethylated styrenes via a visible-light photocatalytic decarboxylation strategy using
Eric D Nacsa et al.
Journal of the American Chemical Society, 140(9), 3322-3330 (2018-02-06)
Nature routinely engages alcohols as leaving groups, as DNA biosynthesis relies on the removal of water from ribonucleoside diphosphates by a radical-mediated "spin-center shift" (SCS) mechanism. Alcohols, however, remain underused as alkylating agents in synthetic chemistry due to their low
Amandeep Arora et al.
Organic letters, 18(16), 3996-3999 (2016-08-06)
The 2-azolyl radical, generated from 2-bromoazoles via photocatalysis, is a powerful intermediate for the intermolecular arylation of unmodified (hetero)arenes. The reaction is characterized by mild conditions, operational simplicity, tolerance toward functional and sterically demanding groups, broad scope, and anti-Minisci selectivity.
Facile synthesis and complete characterization of homoleptic and heteroleptic cyclometalated Iridium(III) complexes for photocatalysis
Singh A, et al.
Journal of Organometallic Chemistry, 776, 51-59 (2015)
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service![(Ir[dF(CF3)ppy]2(dtbpy))PF6](/deepweb/assets/sigmaaldrich/product/structures/982/913/02dd8ddd-6deb-40a0-ab9b-07b18f1abb09/640/02dd8ddd-6deb-40a0-ab9b-07b18f1abb09.png)
![Tris[2-phenylpyridinato-C2,N]iridium(III) 97%](/deepweb/assets/sigmaaldrich/product/structures/167/234/658d0b76-d31d-4fd5-8041-e04e207227c9/640/658d0b76-d31d-4fd5-8041-e04e207227c9.png)
![[Ir(dtbbpy)(ppy)2]PF6](/deepweb/assets/sigmaaldrich/product/structures/158/329/2544d673-d267-4aa1-8f46-2652aad4bfa0/640/2544d673-d267-4aa1-8f46-2652aad4bfa0.png)


![Ir[dF(t-Bu)-ppy]3](/deepweb/assets/sigmaaldrich/product/structures/254/294/d0fb19e5-05b2-4c1b-990b-a99fa60b3e73/640/d0fb19e5-05b2-4c1b-990b-a99fa60b3e73.png)
![[Ir(dF(Me)ppy)2(dtbbpy)]PF6](/deepweb/assets/sigmaaldrich/product/structures/150/099/7c2dfa31-39f4-4cca-aee5-86d4a89fea78/640/7c2dfa31-39f4-4cca-aee5-86d4a89fea78.png)
