All Photos(3)
About This Item
Linear Formula:
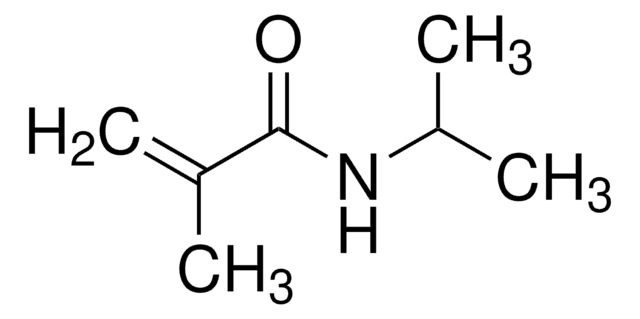
H2C=CHCONHCH(CH3)2
CAS Number:
Molecular Weight:
113.16
EC Number:
MDL number:
UNSPSC Code:
12162002
PubChem Substance ID:
NACRES:
NA.23
Recommended Products
Assay
≥99%
form
flakes
bp
89-92 °C/2 mmHg (lit.)
mp
60-63 °C (lit.)
63-67 °C
storage temp.
2-8°C
SMILES string
CC(C)NC(=O)C=C
InChI
1S/C6H11NO/c1-4-6(8)7-5(2)3/h4-5H,1H2,2-3H3,(H,7,8)
InChI key
QNILTEGFHQSKFF-UHFFFAOYSA-N
Looking for similar products? Visit Product Comparison Guide
General description
N-Isopropylacrylamide (NIPAM) is a biocompatible monomeric unit that can be used in the formation of stimuli responsive polymers due to its temperature sensitive properties. These properties include temperature based volumetric and phase changes, which change when the temperature of the solution reaches a lower critical solution temperature (LCST).
Application
NIPAM can be used to prepare poly(NIPAM) for a variety of applications such as tissue engineering, cell culture, biomedical coating, drug delivery, and muscle regeneration.
N-Isopropylacrylamide can also be used as a key component in:
N-Isopropylacrylamide can also be used as a key component in:
- The synthesis of self-powered multifunctional organic hydrogel based on poly(acrylic acid-N-isopropyl acrylamide) for flexible sensing devices.
- The development of a new type of flexible and stable gel electrolyte for aqueous Zn-MnO2 batteries.
- The preparation of poly(N-isopropylacrylamide/itaconic acid) (PNIPAM/IA) copolymeric hydrogels for drug delivery applications.
- The synthesis of poly(N-isopropylacrylamide) (PNIPAM) polymer for the development of a new class of polymer-grafted semiconductor devices.
Signal Word
Danger
Hazard Statements
Precautionary Statements
Hazard Classifications
Acute Tox. 4 Oral - Eye Dam. 1
Storage Class Code
11 - Combustible Solids
WGK
WGK 3
Certificates of Analysis (COA)
Search for Certificates of Analysis (COA) by entering the products Lot/Batch Number. Lot and Batch Numbers can be found on a product’s label following the words ‘Lot’ or ‘Batch’.
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Customers Also Viewed
Nhung H A Nguyen et al.
Nanoscale research letters, 12(1), 571-571 (2017-10-21)
The most challenging task in the preparation of magnetic poly(N-isopropylacrylamide) (Fe
Bio-Instructive Scaffolds for Muscle Regeneration: NonCrosslinked Polymers
Bio-Instructive Scaffolds for Musculoskeletal Tissue Engineering and Regenerative Medicine, 34(9), 161-186 (2017)
Yamin Li et al.
Biomacromolecules, 13(11), 3877-3886 (2012-09-28)
Polymeric drug nanocarriers integrated with diagnostic and sensing functions are capable of in situ monitoring the biodistribution of chemotherapeutic drugs and imaging/contrasting agents, which enables the establishment of image-guided personalized cancer therapeutic protocols. Responsive multifunctional theranostic nanocarriers possessing external stimuli-tunable
Characterisation of biomedical coatings
Coatings for Biomedical Applications, 32(5), 176-220 (2012)
Firdaus Yhaya et al.
Macromolecular rapid communications, 33(21), 1868-1874 (2012-08-24)
A block copolymer based on poly(N-isopropyl acrylamide) (PNIPAAm) and a block with a statistical distribution of poly(2-hydroxyethyl acrylate) (PHEA) and repeating unit with carrying β-cyclodextrin was prepared via reversible addition-fragmentation chain transfer (RAFT) polymerization and click reaction. Addition of poly(2-hydroxyethyl
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service