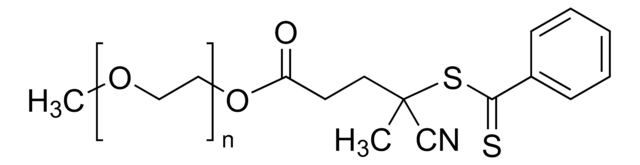
722995
4-Cyano-4-(phenylcarbonothioylthio)pentanoic acid
Synonym(s):
4-Cyano-4-(thiobenzoylthio)pentanoic acid
Sign Into View Organizational & Contract Pricing
All Photos(1)
About This Item
Empirical Formula (Hill Notation):
C13H13NO2S2
CAS Number:
Molecular Weight:
279.38
MDL number:
UNSPSC Code:
12352100
PubChem Substance ID:
NACRES:
NA.23
Recommended Products
form
powder
mp
94-98 °C
storage temp.
2-8°C
SMILES string
CC(CCC(O)=O)(SC(=S)c1ccccc1)C#N
InChI
1S/C13H13NO2S2/c1-13(9-14,8-7-11(15)16)18-12(17)10-5-3-2-4-6-10/h2-6H,7-8H2,1H3,(H,15,16)
InChI key
YNKQCPNHMVAWHN-UHFFFAOYSA-N
Related Categories
General description
4-Cyano-4-(phenylcarbonothioylthio)pentanoic acid is asulfur-based chain transfer agent that provides a high degree of control forliving radical polymerization.
Application
RAFT agent for controlled radical polymerization; especially suited for the polymerization of methacrylate and methacrylamide monomers.
Choose from one of the most recent versions:
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Customers Also Viewed
Bartlomiej Kalaska et al.
Translational research : the journal of laboratory and clinical medicine, 177, 98-112 (2016-07-28)
The parenteral anticoagulants may cause uncontrolled and life-threatening bleeding. Protamine, the only registered heparin antidote, is partially effective against low-molecular weight heparins, completely ineffective against fondaparinux and may cause unacceptable toxicity. Therefore, we aimed to develop a synthetic compound for
Sieun Kim et al.
Biomacromolecules, 21(8), 3026-3037 (2020-07-17)
Charge anisotropy or the presence of charge patches at protein surfaces has long been thought to shift the coacervation curves of proteins and has been used to explain the ability of some proteins to coacervate on the "wrong side" of
Anti-biofouling phosphorylated HEMA and PEGMA block copolymers show high affinity to hydroxyapatite.
Xinnan Cui et al.
Colloids and surfaces. B, Biointerfaces, 160, 289-296 (2017-09-26)
Four types of phosphorylated 2-hydroxyethyl methacrylate and poly(ethylene glycol) methyl ether methacrylate (PEGMA) block copolymers were synthesized by reversible addition fragmentation chain transfer (RAFT) polymerization and post-phosphorylation. These polymers were composed of different phosphate segments and similar PEG brushes. Polymers
Synthesis of Block Copolymer Brush by RAFT and Click Chemistry and Its Self-Assembly as a Thin Film.
Hajeeth Thankappan et al.
Molecules (Basel, Switzerland), 25(20) (2020-10-22)
A well-defined block copolymer brush poly(glycidyl methacrylate)-graft-(poly(methyl methacrylate)-block- poly(oligo(ethylene glycol) methyl ether methacrylate)) (PGMA-g-(PMMA-b-POEGMA)) is synthesized via grafting from an approach based on a combination of click chemistry and reversible addition-fragmentation chain transfer (RAFT) polymerization. The resulting block copolymer brushes
Chunyun Wang et al.
Drug delivery, 27(1), 344-357 (2020-02-25)
Stimuli-responsive drug delivery systems (DDSs) are expected to realize site-specific drug release and kill cancer cells selectively. In this study, a pH-responsive micelle was designed utilizing the pH-sensitivity of borate bonds formed between dopamine and boronic acid. First, methyl (polyethylene
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service![4-Cyano-4-[(dodecylsulfanylthiocarbonyl)sulfanyl]pentanoic acid 97% (HPLC)](/deepweb/assets/sigmaaldrich/product/structures/204/925/30ae6ca0-5b0b-4963-a061-7e5e3d1a85af/640/30ae6ca0-5b0b-4963-a061-7e5e3d1a85af.png)


![2-[[(2-Carboxyethyl)sulfanylthiocarbonyl]-sulfanyl]propanoic acid](/deepweb/assets/sigmaaldrich/product/structures/427/606/b02310e2-102e-4324-b09d-e4c0de4fab2c/640/b02310e2-102e-4324-b09d-e4c0de4fab2c.png)



![4-Cyano-4-[(dodecylsulfanylthiocarbonyl)sulfanyl]pentanol](/deepweb/assets/sigmaaldrich/product/structures/839/520/64c23004-f340-460f-a379-8670a35d0433/640/64c23004-f340-460f-a379-8670a35d0433.png)