358002
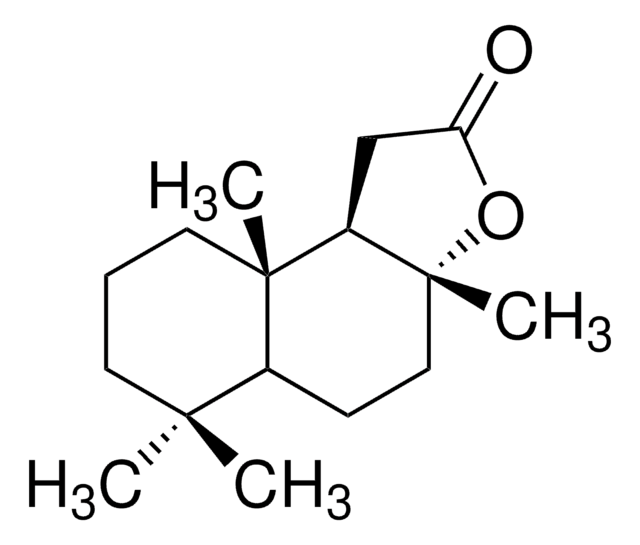
(3aR)-(+)-Sclareolide
97%
Synonym(s):
(+)-Norambreinolide
Sign Into View Organizational & Contract Pricing
All Photos(1)
About This Item
Empirical Formula (Hill Notation):
C16H26O2
CAS Number:
Molecular Weight:
250.38
EC Number:
MDL number:
UNSPSC Code:
12352005
PubChem Substance ID:
NACRES:
NA.22
Recommended Products
Quality Level
Assay
97%
form
powder
optical activity
[α]20/D +47°, c = 2% in chloroform
mp
124-126 °C (lit.)
functional group
ester
SMILES string
[H][C@@]12CC[C@@]3(C)OC(=O)C[C@]3([H])[C@@]1(C)CCCC2(C)C
InChI
1S/C16H26O2/c1-14(2)7-5-8-15(3)11(14)6-9-16(4)12(15)10-13(17)18-16/h11-12H,5-10H2,1-4H3/t11-,12+,15-,16+/m0/s1
InChI key
IMKJGXCIJJXALX-SHUKQUCYSA-N
Looking for similar products? Visit Product Comparison Guide
Related Categories
General description
Sclareolide is a diterpenoid compound mainly used in the perfume industry for its amber like odor.
Application
(3aR)-(+)-Sclareolide may be used in the total syntheses of bioactive compounds such as (+)-chloropuupehenone, (+)-chloropuupehenol, cyslabdan, acuminolide and 17-O-acetylacuminolide. It may also be used in the preparation of γ-bicyclohomofarnesal, an ambergris odorant.
Storage Class Code
11 - Combustible Solids
WGK
WGK 2
Flash Point(F)
Not applicable
Flash Point(C)
Not applicable
Personal Protective Equipment
dust mask type N95 (US), Eyeshields, Gloves
Choose from one of the most recent versions:
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Customers Also Viewed
Synthesis and Structural Revision of Cyslabdan.
Ohtawa M, et al.
Chemical & Pharmaceutical Bulletin, 64(9), 1370-1377 (2016)
Natural sesquiterpenoids.
Fraga BM.
Natural Product Reports, 20(4), 392-413 (2003)
Synthesis of Acuminolide and 17-O-Acetylacuminolide from (+)-Sclareolide.
Zoretic PA, et al.
The Journal of Organic Chemistry, 63(4), 1156-1161 (1998)
Superacid cyclization of homo-and bishomoisoprenoid acids.
Vlad PF, et al.
Chemistry of Heterocyclic Compounds, 27(3), 246-249 (1991)
Total syntheses of (+)-chloropuupehenone and (+)-chloropuupehenol and their analogues and evaluation of their bioactivities.
Hua DH, et al.
The Journal of Organic Chemistry, 69(18), 6065-6078 (2004)
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service