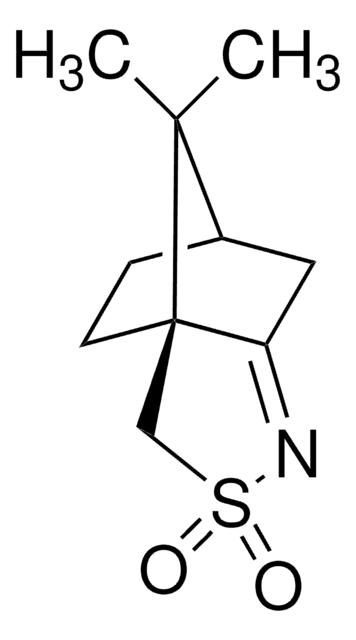
349003
(1R)-(−)-(10-Camphorsulfonyl)oxaziridine
Synonym(s):
(1R)-(−)-(Camphorylsulfonyl)oxaziridine, (1R)-(−)-2,N-Epoxy-exo-10,2-bornanesultam
Sign Into View Organizational & Contract Pricing
All Photos(1)
About This Item
Empirical Formula (Hill Notation):
C10H15NO3S
CAS Number:
Molecular Weight:
229.30
Beilstein:
6274369
MDL number:
UNSPSC Code:
12352005
PubChem Substance ID:
NACRES:
NA.22
Recommended Products
form
powder
Quality Level
optical activity
[α]20/D −44°, c = 2.2 in chloroform
storage temp.
2-8°C
SMILES string
[H][C@@]12CC[C@@]3(CS(=O)(=O)N4OC34C1)C2(C)C
InChI
1S/C10H15NO3S/c1-8(2)7-3-4-9(8)6-15(12,13)11-10(9,5-7)14-11/h7H,3-6H2,1-2H3/t7-,9-,10?,11?/m1/s1
InChI key
GBBJBUGPGFNISJ-JRNSMZEGSA-N
Application
(1R)-(-)-(10-Camphorsulfonyl)oxaziridine may be used as an oxidant to prepare (S)-(-)-vasicinone via asymmetric oxidation of deoxyvasicinone.
Reactant involved in:
Used in impregnated silica nanoparticles for removal of sulfur mustard from wastewater
Used to modify blebbistatin for investigations of myosin inhibitor design
- Asymmetric synthesis of proton pump inhibitors
- Asymmetric synthesis of polyhydroxylated pyrrolidines
- Diastereoselective hydroxylation of chlorophylls a and b enolate anions
Used in impregnated silica nanoparticles for removal of sulfur mustard from wastewater
Used to modify blebbistatin for investigations of myosin inhibitor design
Storage Class Code
11 - Combustible Solids
WGK
WGK 3
Flash Point(F)
Not applicable
Flash Point(C)
Not applicable
Choose from one of the most recent versions:
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Customers Also Viewed
Synthesis of Optically Active Vasicinone Based on Intramolecular Aza-Wittig Reaction and Asymmetric Oxidation1.
Eguchi S, et al.
The Journal of Organic Chemistry, 61(21), 7316-7319 (1996)
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service





![(Ir[dF(CF3)ppy]2(dtbpy))PF6](/deepweb/assets/sigmaaldrich/product/structures/982/913/02dd8ddd-6deb-40a0-ab9b-07b18f1abb09/640/02dd8ddd-6deb-40a0-ab9b-07b18f1abb09.png)