All Photos(1)
About This Item
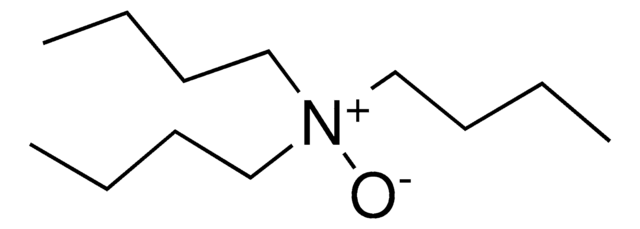
Linear Formula:
(CH3)3N(O)
CAS Number:
Molecular Weight:
75.11
EC Number:
MDL number:
UNSPSC Code:
12352116
PubChem Substance ID:
NACRES:
NA.22
Recommended Products
Assay
95%
reaction suitability
reagent type: oxidant
mp
220-222 °C (lit.)
SMILES string
C[N+](C)(C)[O-]
InChI
1S/C3H9NO/c1-4(2,3)5/h1-3H3
InChI key
UYPYRKYUKCHHIB-UHFFFAOYSA-N
Looking for similar products? Visit Product Comparison Guide
General description
Trimethylamine N-oxide is an organic compound that belongs to the class of amine oxides. It is generally found in the tissues of marine organisms, wherein it helps protect them from harsh conditions like salinity, hydrostatic pressure, temperature, and high urea.
Application
Trimethylamine N-oxide can be used:
- As a demetallation and decarbonylation reagent for organometallic compounds.
- To prepare azomethine ylide by reaction with lithium di-isopropylamide. This, in turn, may be reacted with simple alkenes to obtain corresponding pyrrolidines.
- To mediate the conversion of thiols to disulfides.
Signal Word
Warning
Hazard Statements
Precautionary Statements
Hazard Classifications
Acute Tox. 4 Inhalation - Acute Tox. 4 Oral
Storage Class Code
11 - Combustible Solids
WGK
WGK 3
Personal Protective Equipment
dust mask type N95 (US), Eyeshields, Gloves
Certificates of Analysis (COA)
Search for Certificates of Analysis (COA) by entering the products Lot/Batch Number. Lot and Batch Numbers can be found on a product’s label following the words ‘Lot’ or ‘Batch’.
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Customers Also Viewed
J Klysik et al.
FEBS letters, 333(3), 261-267 (1993-11-01)
The ability of the Escherichia coli single-stranded DNA-binding protein (SSB) to recognize structural features associated with intramolecular triplex formation in oligopurine.oligopyrimidine (pur.pyr) inserts in recombinant plasmids was evaluated. The SSB protein binds to supercoiled plasmids and causes a site-preferential increase
Site selectivity studies on heterobimetallic complexes: substitution reactions of (.eta.5-C5H5)MM'(CO)8 (M = Mo, W; M' = Mn, Re)
Ingham WL and Coville NJ
Inorganic Chemistry, 31(20), 4084-4090 (1992)
Hydride ion transfer from carbon-hydrogen bonds to carbon disulfide, carbonyl sulfide, and carbon dioxide. Synthesis, reactivity, and structure of manganese complexes (η 5-C6MenH7-n) Mn (CO) LL'
Snyder DB, et al.
Journal of the American Chemical Society, 115(15), 6718-6729 (1993)
Vanessa Siegmund et al.
Scientific reports, 6, 39291-39291 (2016-12-17)
Spontaneous isopeptide bond formation, a stabilizing posttranslational modification that can be found in gram-positive bacterial cell surface proteins, has previously been used to develop a peptide-peptide ligation technology that enables the polymerization of tagged-proteins catalyzed by SpyLigase. Here we adapted
Marina Spörrer et al.
EBioMedicine, 44, 502-515 (2019-05-13)
Missense mutations in keratin 5 and 14 genes cause the severe skin fragility disorder epidermolysis bullosa simplex (EBS) by collapsing of the keratin cytoskeleton into cytoplasmic protein aggregates. Despite intense efforts, no molecular therapies are available, mostly due to the
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service