302589
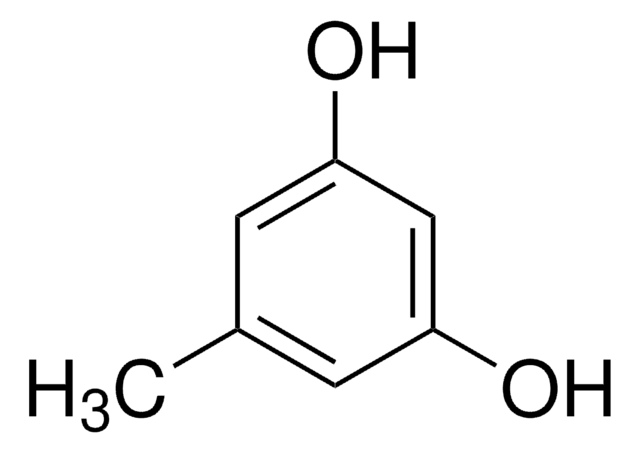
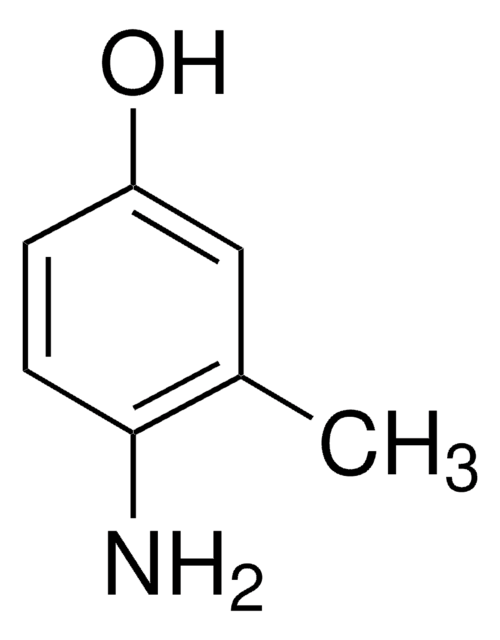
2-Methylresorcinol
98%
Synonym(s):
2,6-Dihydroxytoluene
Sign Into View Organizational & Contract Pricing
All Photos(2)
About This Item
Linear Formula:
CH3C6H3(OH)2
CAS Number:
Molecular Weight:
124.14
Beilstein:
2042177
EC Number:
MDL number:
UNSPSC Code:
12352100
PubChem Substance ID:
NACRES:
NA.22
Recommended Products
Quality Level
Assay
98%
form
solid
bp
264 °C (lit.)
mp
114-120 °C (lit.)
SMILES string
Cc1c(O)cccc1O
InChI
1S/C7H8O2/c1-5-6(8)3-2-4-7(5)9/h2-4,8-9H,1H3
InChI key
ZTMADXFOCUXMJE-UHFFFAOYSA-N
Looking for similar products? Visit Product Comparison Guide
General description
The reaction between 2-methylresorcinol and 2-alkenals was studied to investigate the scavenging ability of m-diphenols for the 2-alkenals formed during lipid oxidation.
Application
2-Methylresorcinol was used in the synthesis of:
- C-5-bromo-2-hydroxyphenylcalix[4]-2-methylresorcinarene
- tripyrrane analogs
- series of novel aromatic benziporphyrins
Signal Word
Danger
Hazard Statements
Precautionary Statements
Hazard Classifications
Acute Tox. 3 Oral - Aquatic Acute 1 - Eye Dam. 1 - Skin Sens. 1B
Storage Class Code
6.1C - Combustible acute toxic Cat.3 / toxic compounds or compounds which causing chronic effects
WGK
WGK 3
Flash Point(F)
275.0 °F - closed cup
Flash Point(C)
135 °C - closed cup
Personal Protective Equipment
dust mask type N95 (US), Eyeshields, Gloves
Choose from one of the most recent versions:
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Customers Also Viewed
Kae Miyake et al.
Chemical communications (Cambridge, England), (2)(2), 178-179 (2004-01-23)
Acid catalyzed condensation of resorcinol or 2-methylresorcinol with 2 equiv. of an acetoxymethylpyrrole gave bis(pyrrolylmethyl)benzene derivatives in moderate yields; these afforded a series of novel aromatic benziporphyrins using the MacDonald "3 + 1" methodology.
Hamza M Abosadiya et al.
Molecules (Basel, Switzerland), 18(11), 13369-13384 (2013-11-01)
C-5-bromo-2-hydroxyphenylcalix[4]-2-methylresorcinarene (I) was synthesized by cyclocondensation of 5-bromo-2-hydroxybenzaldehyde and 2-methylresorcinol in the presence of concentrated HCl. Compound I was characterized by infrared and nuclear magnetic resonance spectroscopic data. X-ray analysis showed that this compound crystallized in a triclinic system with
Timothy D Lash et al.
The Journal of organic chemistry, 76(15), 6295-6308 (2011-06-23)
Tripyrrane analogues were prepared by reacting resorcinol or 2-methylresorcinol with 2 equiv of an acetoxymethylpyrrole in the presence of p-toluenesulfonic acid and calcium chloride. Following removal of the benzyl ester protective groups, the resorcinol-derived benzitripyrrane was reacted with a pyrrole
Francisco J Hidalgo et al.
Food chemistry, 160, 118-126 (2014-05-07)
The reaction between m-diphenols (resorcinol, 2-methylresorcinol, 2,5-dimethylresorcinol, 3-methylphenol, orcinol, and phloroglucinol) and 2-alkenals (2-pentenal and 2-octenal) was studied in an attempt to understand the chemical pathways involved in the scavenging ability of m-diphenols for the 2-alkenals produced as a consequence
O K Davydova et al.
Mikrobiologiia, 75(5), 654-661 (2006-11-10)
We established that chemical analogues of alkylhydroxybenzenes (AHB), belonging to alkylresorcinols and functioning as microbial autoregulatory d1 factors, enhance the UV resistance of various DNA molecules of different origin and conformation. These include the linear DNA of the lambda phage
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service