All Photos(1)
About This Item
Linear Formula:
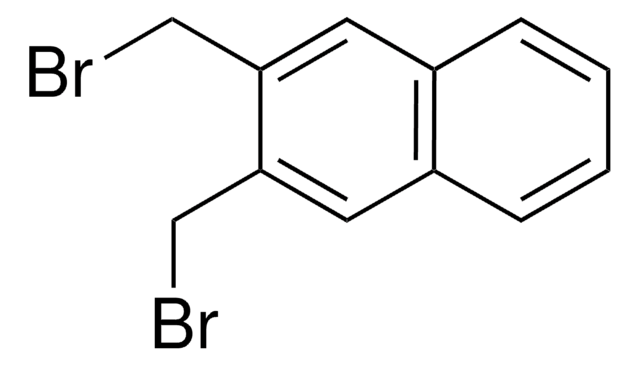
C10H6(CH2Br)2
CAS Number:
Molecular Weight:
314.02
MDL number:
UNSPSC Code:
12352100
PubChem Substance ID:
Recommended Products
Assay
98%
form
crystals
mp
131-133 °C (lit.)
SMILES string
BrCc1cccc2cccc(CBr)c12
InChI
1S/C12H10Br2/c13-7-10-5-1-3-9-4-2-6-11(8-14)12(9)10/h1-6H,7-8H2
InChI key
GCZOMCDXYFMAGP-UHFFFAOYSA-N
General description
Two-photon chemistry of 1,8-bis(bromomethyl)naphthalene has been studied by time-delayed, two-color photolysis and argon ion laser-jet photolysis techniques.
Application
1,8-Bis(bromomethyl)naphthalene has been used in the synthesis of indan-based unusual α-amino acid derivatives under solid-liquid phase-transfer catalysis conditions.
Signal Word
Danger
Hazard Statements
Precautionary Statements
Hazard Classifications
Eye Dam. 1 - Skin Corr. 1B
Storage Class Code
8A - Combustible corrosive hazardous materials
WGK
WGK 3
Flash Point(F)
Not applicable
Flash Point(C)
Not applicable
Personal Protective Equipment
dust mask type N95 (US), Eyeshields, Gloves
Certificates of Analysis (COA)
Search for Certificates of Analysis (COA) by entering the products Lot/Batch Number. Lot and Batch Numbers can be found on a product’s label following the words ‘Lot’ or ‘Batch’.
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Customers Also Viewed
Two-Photon Laser-Induced Reaction of 1, 8-Bis (halomethyl) naphthalenes from Different Excited States and Transient Targeting of Its Intermediate by Time-Delayed, Two-Color Photolysis and Argon Ion Laser-Jet Photolysis Techniques
Ouchi A, et al.
Journal of the American Chemical Society, 119(3), 592-599 (1997)
S Kotha et al.
The Journal of organic chemistry, 65(5), 1359-1365 (2000-05-18)
Conformationally constrained cyclic alpha-amino acid derivatives were synthesized under solid-liquid phase-transfer catalysis conditions. This methodology involves the bis-alkylation of ethyl isocyanoacetate with various alpha,alpha'-dibromo-o-xylene derivatives [alpha,alpha'-dibromo-o-xylene 5, 2,3-bis(bromomethyl)-1, 4-dimethoxybenzene 6, 1,2-bis(bromomethyl)-4,5-dibromobenzene 7, 2, 3-bis(bromomethyl)naphthalene 8, 1,8-bis(bromomethyl)-naphthalene 9, 6,7-bis(bromomethyl)-2,2-dimethyl-1H-phenalene-1,3(2H)-dione 10, 2
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service