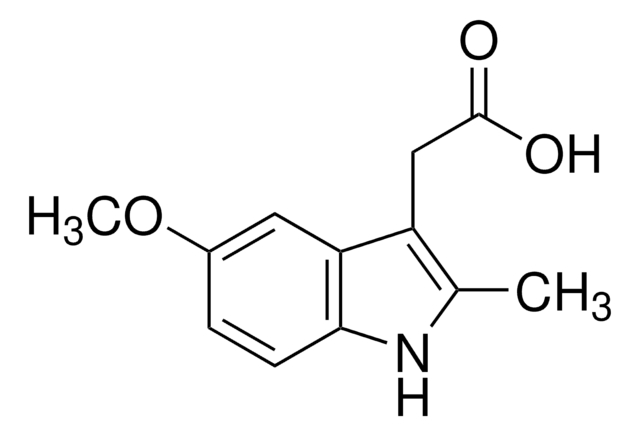
105171
5-Methoxy-2-methyl-3-indoleacetic acid
98%
Synonym(s):
N-Des(4-chlorobenzoyl)indomethacin, NSC 97026, 2-(5-Methoxy-2-methyl-1H-indol-3-yl)acetic acid
Sign Into View Organizational & Contract Pricing
All Photos(3)
About This Item
Empirical Formula (Hill Notation):
C12H13NO3
CAS Number:
Molecular Weight:
219.24
EC Number:
MDL number:
UNSPSC Code:
12352100
PubChem Substance ID:
NACRES:
NA.22
Recommended Products
Quality Level
Assay
98%
mp
161-163 °C (lit.)
solubility
methanol: soluble
functional group
carboxylic acid
SMILES string
COc1ccc2[nH]c(C)c(CC(O)=O)c2c1
InChI
1S/C12H13NO3/c1-7-9(6-12(14)15)10-5-8(16-2)3-4-11(10)13-7/h3-5,13H,6H2,1-2H3,(H,14,15)
InChI key
TXWGINUZLBAKDF-UHFFFAOYSA-N
Looking for similar products? Visit Product Comparison Guide
General description
5-Methoxy-2-methyl-3-indoleacetic acid is hydrolysis product of indomethacin.
Application
5-Methoxy-2-methyl-3-indoleacetic acid was used for quantitative determination of indomethacin and its major impurities in suppository and capsule formulations by HPLC. 5-Methoxy-2-methyl-3-indoleacetic acid was used in a study to develop fast, sensitive and simultaneous determination of metabolites of serotonin using liquid chromatography with mass spectrometric detection.
Storage Class Code
11 - Combustible Solids
WGK
WGK 3
Flash Point(F)
Not applicable
Flash Point(C)
Not applicable
Personal Protective Equipment
dust mask type N95 (US), Eyeshields, Gloves
Choose from one of the most recent versions:
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
H Alho et al.
Cell growth & differentiation : the molecular biology journal of the American Association for Cancer Research, 5(9), 1005-1014 (1994-09-01)
A recognition site for benzodiazepines structurally different from that linked to the gamma-aminobutyric acid receptor subtype A or the "central type" benzodiazepine receptor has been located mainly in the outer membranes of mitochondria and designated mitochondrial benzodiazepine receptor (MBR). A
E Kwong et al.
Journal of pharmaceutical sciences, 71(7), 828-830 (1982-07-01)
Indomethacin and its impurities in suppository and capsule formulations were quantitatively determined by HPLC using a reversed-phase, octadecyl column and a mobile phase of methanol-water-acetonitrile-acetic acid (55:35:10:1). Analysis of the suppository formulations provided a mean potency for indomethacin of 103.8%.
Peng Zhang et al.
Acta pharmacologica Sinica, 27(8), 1097-1102 (2006-07-27)
To investigate the biotransformation of indomethacin, the first of the newer nonsteroidal anti-inflammatory drugs, by filamentous fungus and to compare the similarities between microbial transformation and mammalian metabolism of indomethacin. Five strains of Cunninghamella (C elegans AS 3.156, C elegans
R D Johnson et al.
Journal of chromatography. B, Analytical technologies in the biomedical and life sciences, 805(2), 223-234 (2004-05-12)
Toxicological examination of fatal aviation accident victims routinely includes analysis of ethanol levels. However, distinguishing between antemortem ingestion and postmortem microbial formation complicates all positive ethanol results. Development of a single analytical approach to determine concentrations of 5-hydroxytryptophol (5-HTOL) and
J Krzek et al.
Journal of AOAC International, 84(6), 1703-1707 (2002-01-05)
A densitometric method was developed for the identification and determination of indomethacin and its degradation products, 4-chlorobenzoic acid and 5-methoxy-2-methyl-3-indoleacetic acid, in pharmaceuticals. To separate these compounds, silica gel-coated thin-layer chromatography plates and the following mobile phase were used: 2-propanol-25%
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service