SAB1300492
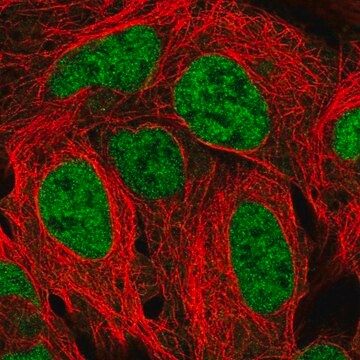
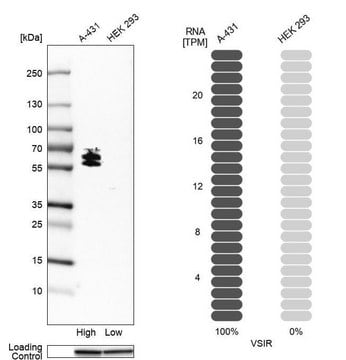
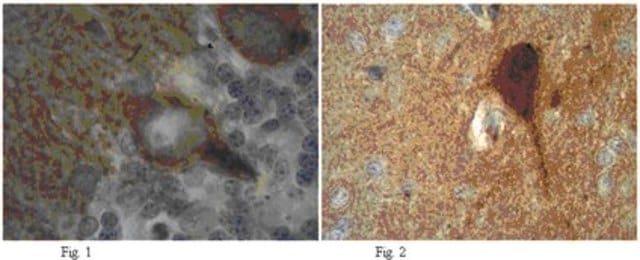
Anti-PTPβ (C-term) antibody produced in rabbit
IgG fraction of antiserum, buffered aqueous solution
Sign Into View Organizational & Contract Pricing
All Photos(1)
About This Item
UNSPSC Code:
12352203
NACRES:
NA.41
Recommended Products
biological source
rabbit
conjugate
unconjugated
antibody form
IgG fraction of antiserum
antibody product type
primary antibodies
clone
polyclonal
form
buffered aqueous solution
species reactivity
human
technique(s)
indirect ELISA: 1:1000
NCBI accession no.
UniProt accession no.
shipped in
dry ice
storage temp.
−20°C
target post-translational modification
unmodified
Gene Information
human ... PTPRB(5787)
General description
Phosphorylation of receptors by protein kinases is a process that can be reversed by a group of enzymes called protein phosphatases. Coordinated control of kinases and phosphatases provides the cell with the capacity to rapidly switch between phosphorylated and dephosphorylated protein states in dynamic response to environmental stimuli. Activation of critical enzymes by kinase phosphorylation alone is not enough to provide adequate regulation – it is the combination with phosphatase dephosphorylation that effectively creates on/off switches to control cellular events. Errors in control, either through kinases or their counterpart phosphatases, can lead to unchecked cell growth attributable to human cancers and developmental disorders. Potential mechanisms to control dephosphorylation include changes in the expression of protein phosphatases, their subcellular localization, phosphorylation of phosphatase catalytic and regulatory subunits and regulation by endogenous phosphatase inhibitors. Most protein phosphatases are not stringently specific for their substrates. Consequently, changes in phosphatase activity may have a broad impact on dephosphorylation and turnover of phosphoproteins that are substrates for different kinases. This may be an important point of control to connect cellular circuitry of interrelated signaling pathways, and to synchronize physiological responses.
Immunogen
PTPBETA (1957-1993)
This antibody is generated from rabbits immunized with a KLH conjugated synthetic peptide selected from the C-terminal region of human PTPbeta.
This antibody is generated from rabbits immunized with a KLH conjugated synthetic peptide selected from the C-terminal region of human PTPbeta.
Biochem/physiol Actions
Protein tyrosine phosphatase, receptor type B (PTPRB), also known as vascular endothelial-protein-tyrosine phosphatase (VE-PTP), might have a role in remodeling of blood vessels and in angiogenesis. It is also important for endothelial cell migration. The protein also controls the phosphorylation state of the angiopoietin receptor, Tie-2. PTPRB may modulate sodium channels by influencing the tyrosine phosphorylation status.
Physical form
Purified polyclonal antibody supplied in PBS with 0.09% (W/V) sodium azide.
Not finding the right product?
Try our Product Selector Tool.
Storage Class Code
10 - Combustible liquids
WGK
nwg
Flash Point(F)
Not applicable
Flash Point(C)
Not applicable
Certificates of Analysis (COA)
Search for Certificates of Analysis (COA) by entering the products Lot/Batch Number. Lot and Batch Numbers can be found on a product’s label following the words ‘Lot’ or ‘Batch’.
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Picomolar concentrations of free zinc(II) ions regulate receptor protein-tyrosine phosphatase ? activity.
Wilson M
The Journal of Biological Chemistry (2012)
Shear stress-induced redistribution of vascular endothelial-protein-tyrosine phosphatase (VE-PTP) in endothelial cells and its role in cell elongation.
Mantilidewi KI
The Journal of Biological Chemistry (2014)
A sodium channel signaling complex: modulation by associated receptor protein tyrosine phosphatase beta.
Ratcliffe CF
Nature Neuroscience (2000)
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service