C5490
C75
≥98% (HPLC), powder
Synonym(s):
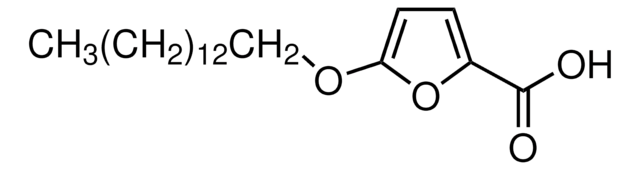
4-Methylene-2-octyl-5-oxotetrahydrofuran-3-carboxylic acid
Sign Into View Organizational & Contract Pricing
All Photos(1)
About This Item
Empirical Formula (Hill Notation):
C14H22O4
CAS Number:
Molecular Weight:
254.32
MDL number:
UNSPSC Code:
41121801
PubChem Substance ID:
NACRES:
NA.77
Recommended Products
Quality Level
Assay
≥98% (HPLC)
form
powder
color
white to beige
solubility
DMSO: 5 mg/mL, clear
storage temp.
2-8°C
SMILES string
CCCCCCCCC1OC(=O)C(=C)C1C(O)=O
InChI
1S/C14H22O4/c1-3-4-5-6-7-8-9-11-12(13(15)16)10(2)14(17)18-11/h11-12H,2-9H2,1H3,(H,15,16)
InChI key
VCWLZDVWHQVAJU-UHFFFAOYSA-N
Application
C75 has been used:
- as a fatty acid synthase (FASN) inhibitor to test its ability in the direct inhibition of FASN to attenuate mammosphere formation as compared to metformin
- as a pharmacological inhibitor to inhibit fatty acid synthesis in glioma stem cells (GSCs)
- as FAS inhibitor in the pre-treatment of Chang cells to inhibit lipogenesis and reverse the senescence induced by hydrogen peroxide
Biochem/physiol Actions
C75 helps to increase the cancer‐killing capability of ionizing radiation.
C75 is a novel, potent synthetic inhibitor of fatty acid synthase (FAS), which is used as a tool for studying fatty acid synthesis in metabolic disorders and cancer.
Storage Class Code
11 - Combustible Solids
WGK
WGK 3
Flash Point(F)
Not applicable
Flash Point(C)
Not applicable
Personal Protective Equipment
dust mask type N95 (US), Eyeshields, Gloves
Choose from one of the most recent versions:
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Customers Also Viewed
Song-Hyo Jin et al.
PLoS neglected tropical diseases, 11(6), e0005687-e0005687 (2017-06-22)
Leprosy is a chronic infectious disease that is caused by the obligate intracellular pathogen Mycobacterium leprae (M.leprae), which is the leading cause of all non-traumatic peripheral neuropathies worldwide. Although both myelinating and non-myelinating Schwann cells are infected by M.leprae in
Differential in radiosensitizing potency of enantiomers of the fatty acid synthase inhibitor C75
Rae C, et al.
Chirality, 29(1), 10-13 (2017)
Virginia Rodriguez et al.
PloS one, 7(6), e39297-e39297 (2012-07-05)
Posttranscriptional modifications are critical for structure and function of tRNAs. Wybutosine (yW) and its derivatives are hyper-modified guanosines found at the position 37 of eukaryotic and archaeal tRNA(Phe). TYW2 is an enzyme that catalyzes α-amino-α-carboxypropyl transfer activity at the third
Satoshi Kimura et al.
Nucleic acids research, 45(22), 12974-12986 (2017-10-27)
Post-transcriptional modifications of ribosomal RNAs (rRNAs) are involved in ribosome biogenesis and fine-tuning of translation. 5-Hydroxycytidine (ho5C), a modification of unknown biogenesis and function, is present at position 2501 of Escherichia coli 23S rRNA. We conducted a genome-wide screen in
Cheulhee Jung et al.
Analytical and bioanalytical chemistry, 408(30), 8583-8591 (2016-04-02)
There are various ways that priming can occur in nucleic acid amplification reactions. While most reactions rely on a primer to initiate amplification, a mechanism for DNA amplification has been developed in which hairpin sequences at the 3' terminus of
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service