700133P
Avanti
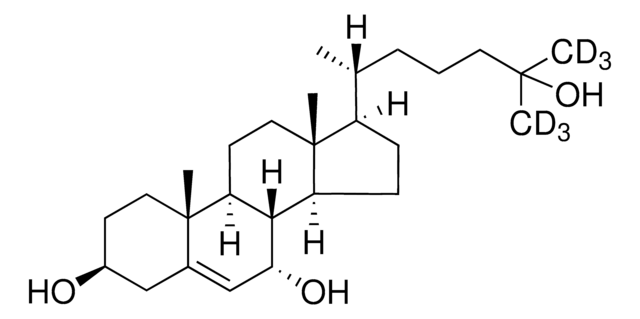
3β,25-OH-7-oxo-5-cholestenoic acid
Avanti Research™ - A Croda Brand 700133P, powder
Synonym(s):
3β,25-dihydroxy-7-oxo-5-cholestenoic acid
Sign Into View Organizational & Contract Pricing
All Photos(2)
About This Item
Empirical Formula (Hill Notation):
C27H42 O5
CAS Number:
Molecular Weight:
446.62
UNSPSC Code:
12352211
NACRES:
NA.25
Recommended Products
Assay
>99% (TLC)
form
powder
packaging
pkg of 1 × 1 mg (700133P-1mg)
manufacturer/tradename
Avanti Research™ - A Croda Brand 700133P
shipped in
dry ice
storage temp.
−20°C
SMILES string
O[C@H](C1)CC[C@](C1=C[C@H]2O)(C)C3C2C(CC[C@@H]4[C@H](C)CCCC(C)(O)CO)[C@]4(C)CC3
General description
3β,25-OH-7-oxo-5-cholestenoic acid is a cholesterol derivative and an oxysterol derived from cholesterol. The synthesis of CA occurs in mitochondria and the enzyme sterol 27-hydroxylase (CYP27A1) catalyzes the step.
Biochem/physiol Actions
Cholestenoic acid (CA) serves as a ligand to nuclear receptor and may be neurotoxic or neuroprotective based on their structural features. Defects in the enzymes catalyzing the formation of cholestenoic acids are implicated in disorders related to motor neuron degeneration.
Packaging
5 mL Amber Glass Screw Cap Vial (700133P-1mg)
Legal Information
Avanti Research is a trademark of Avanti Polar Lipids, LLC
Storage Class Code
11 - Combustible Solids
Certificates of Analysis (COA)
Search for Certificates of Analysis (COA) by entering the products Lot/Batch Number. Lot and Batch Numbers can be found on a product’s label following the words ‘Lot’ or ‘Batch’.
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Cholestenoic acid is a prognostic biomarker in acute respiratory distress syndrome.
Jennifer H Madenspacher et al.
The Journal of allergy and clinical immunology, 143(1), 440-442 (2018-10-09)
Spyridon Theofilopoulos et al.
The Journal of clinical investigation, 124(11), 4829-4842 (2014-10-02)
Cholestenoic acids are formed as intermediates in metabolism of cholesterol to bile acids, and the biosynthetic enzymes that generate cholestenoic acids are expressed in the mammalian CNS. Here, we evaluated the cholestenoic acid profile of mammalian cerebrospinal fluid (CSF) and
Peter J Crick et al.
The Journal of steroid biochemistry and molecular biology, 195, 105475-105475 (2019-09-22)
While the presence and abundance of the major oxysterols and cholestenoic acids in the circulation is well established, minor cholesterol metabolites may also have biological importance and be of value to investigate. In this study by observing the metabolism of
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service