E26258
Ethylene carbonate
98%
Synonym(s):
1,3-Dioxolan-2-one
Sign Into View Organizational & Contract Pricing
All Photos(4)
About This Item
Empirical Formula (Hill Notation):
C3H4O3
CAS Number:
Molecular Weight:
88.06
Beilstein:
106249
EC Number:
MDL number:
UNSPSC Code:
12352100
PubChem Substance ID:
NACRES:
NA.22
Recommended Products
vapor density
3.04 (vs air)
vapor pressure
0.02 mmHg ( 36.4 °C)
Assay
98%
bp
243-244 °C/740 mmHg (lit.)
mp
35-38 °C (lit.)
density
1.321 g/mL at 25 °C (lit.)
SMILES string
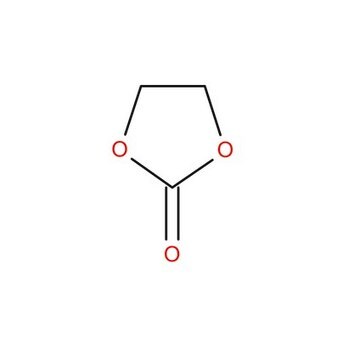
O=C1OCCO1
InChI
1S/C3H4O3/c4-3-5-1-2-6-3/h1-2H2
InChI key
KMTRUDSVKNLOMY-UHFFFAOYSA-N
Looking for similar products? Visit Product Comparison Guide
Related Categories
Application
Ethylene carbonate has been used:
- In the synthesis of aliphatic polyurethanes using diamines and diols.
- As a precursor for ring-opening polymerization using KOH as initiator.
- As a reactant in the preparation of dimethyl carbonate, glycerol carbonate by transesterification reaction.
related product
Product No.
Description
Pricing
Signal Word
Warning
Hazard Statements
Precautionary Statements
Hazard Classifications
Acute Tox. 4 Oral - Eye Irrit. 2 - STOT RE 2 Oral
Target Organs
Kidney
Storage Class Code
11 - Combustible Solids
WGK
WGK 1
Flash Point(F)
289.4 °F - closed cup
Flash Point(C)
143 °C - closed cup
Personal Protective Equipment
dust mask type N95 (US), Eyeshields, Gloves
Choose from one of the most recent versions:
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Customers Also Viewed
Ring-opening polymerization of ethylene carbonate and depolymerization of poly (ethylene oxide-co-ethylene carbonate).
Lee J-C and Litt MH
Macromolecules, 33(5), 1618-1627 (2000)
Kinetics of the production of glycerol carbonate by transesterification of glycerol with dimethyl and ethylene carbonate using potassium methoxide, a highly active catalyst.
Esteban J, et al.
Fuel Processing Technology, 138(5), 243-251 (2015)
Synthesis of dimethyl carbonate from transesterification of ethylene carbonate with methanol using immobilized ionic liquid on commercial silica.
Kim K-H, et al.
Korean Journal of Chemical Engineering, 27(5), 1441-1445 (2010)
Sang-Jae Park et al.
Journal of the American Chemical Society, 137(7), 2565-2571 (2015-02-04)
Here we describe a class of electric-conducting polymers that conduct electrons via the side chain π-π stacking. These polymers can be designed and synthesized with different chemical moieties to perform different functions, extremely suitable as a conductive polymer binder for
Synthesis of dimethyl carbonate by transesterification of ethylene carbonate over activated dawsonites.
Georgiana Stoica et al.
ChemSusChem, 2(4), 301-304 (2009-02-07)
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service