All Photos(1)
About This Item
Empirical Formula (Hill Notation):
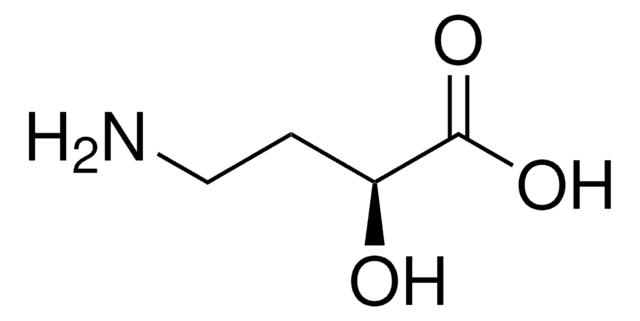
C4H9NO3
CAS Number:
Molecular Weight:
119.12
MDL number:
UNSPSC Code:
12352116
PubChem Substance ID:
NACRES:
NA.22
Recommended Products
Assay
97%
optical activity
[α]20/D +20.0°, c = 1.7 in H2O
mp
207-212 °C
functional group
amine
carboxylic acid
hydroxyl
SMILES string
NC[C@@H](O)CC(O)=O
InChI
1S/C4H9NO3/c5-2-3(6)1-4(7)8/h3,6H,1-2,5H2,(H,7,8)/t3-/m0/s1
InChI key
YQGDEPYYFWUPGO-VKHMYHEASA-N
Related Categories
General description
(S)-(+)-4-Amino-3-hydroxybutyric acid [(S)-GABOB] can be prepared starting from ethyl (S)-4-chloro-3-hydroxybutyrate.
Storage Class Code
11 - Combustible Solids
WGK
WGK 3
Flash Point(F)
Not applicable
Flash Point(C)
Not applicable
Personal Protective Equipment
dust mask type N95 (US), Eyeshields, Gloves
Choose from one of the most recent versions:
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
[Resolution of (3RS)-4-amino-3-hydroxybutanoic acid (author's transl)].
S Miyamoto et al.
Yakugaku zasshi : Journal of the Pharmaceutical Society of Japan, 99(6), 642-646 (1979-06-01)
Different efficacies of d- and l-gamma-amino-beta-hydroxybutyric acids in GABA receptor and transport test systems.
E Roberts et al.
The Journal of neuroscience : the official journal of the Society for Neuroscience, 1(2), 132-140 (1981-02-01)
Short synthesis of (R)-and (S)-4-amino-3-hydroxybutyric acid (GABOB).
Tiecco M, et al.
Synthesis, 2005(04), 579-582 (2005)
J Takahara et al.
Hormone and metabolic research = Hormon- und Stoffwechselforschung = Hormones et metabolisme, 12(1), 31-34 (1980-01-01)
Healthy male volunteers injected subcutaneously with 200 mg L-GABOB showed no significant changes in plasma GH, prolactin and cortisol levels. On the other hand, an intrathecal injection of 300 mg D, L-GABOB to cerebrovascular patients caused significant increases in plasma
G Bonardi et al.
Arzneimittel-Forschung, 31(11), 1910-1913 (1981-01-01)
1. Serum levels of DL-gamma-amino-beta-hydroxybutyric acid-1-14C (DL-GABOB-1-14C) in the rat (50 mg/kg) were quite similar after single i.v. and p.o. doses. Also the disappearance from the serum was similar with both administration routes. Within 6 days after p.o. treatment with
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service