511188
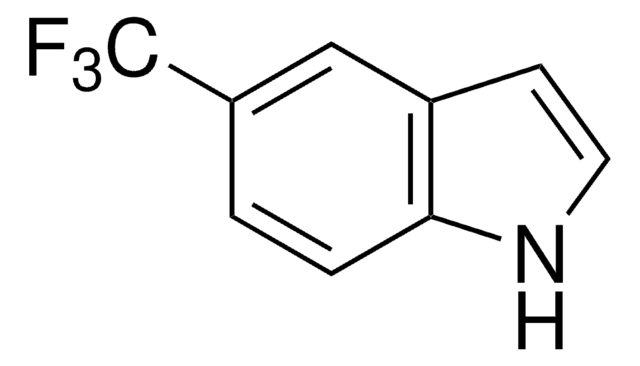
Methyl indole-5-carboxylate
99%
Synonym(s):
Indole-5-carboxylic acid, methyl ester
Sign Into View Organizational & Contract Pricing
All Photos(1)
About This Item
Empirical Formula (Hill Notation):
C10H9NO2
CAS Number:
Molecular Weight:
175.18
MDL number:
UNSPSC Code:
12352100
PubChem Substance ID:
NACRES:
NA.22
Recommended Products
Quality Level
Assay
99%
mp
126-128 °C (lit.)
SMILES string
COC(=O)c1ccc2[nH]ccc2c1
InChI
1S/C10H9NO2/c1-13-10(12)8-2-3-9-7(6-8)4-5-11-9/h2-6,11H,1H3
InChI key
DRYBMFJLYYEOBZ-UHFFFAOYSA-N
General description
Methyl indole-5-carboxylate (Methyl 1H-indole-5-carboxylate), a substituted 1H-indole, can be prepared by the esterification of indole-5-carboxylic acid. Its efficacy as a substrate for indigoid generation has been assessed.
Application
Methyl indole-5-carboxylate may be used as a reactant in the following processes:
- biosynthesis of inhibitors of protein kinases
- metal-free Friedel-Crafts alkylation
- preparation of diphenylsulfonium ylides from Martin′s sulfurane
- cross dehydrogenative coupling reactions
- synthesis of indirubin derivatives
- preparation of aminoindolylacetates
Methyl indole-5-carboxylate may be used in the preparation of:
- methyl indoline-5-carboxylate
- 1H-indole-5-carbohydrazide
- dimethyl 1H-indole-3,5-dicarboxylate
Signal Word
Warning
Hazard Statements
Precautionary Statements
Hazard Classifications
Eye Irrit. 2 - Skin Irrit. 2 - STOT SE 3
Target Organs
Respiratory system
Storage Class Code
11 - Combustible Solids
WGK
WGK 3
Flash Point(F)
Not applicable
Flash Point(C)
Not applicable
Personal Protective Equipment
dust mask type N95 (US), Eyeshields, Gloves
Certificates of Analysis (COA)
Search for Certificates of Analysis (COA) by entering the products Lot/Batch Number. Lot and Batch Numbers can be found on a product’s label following the words ‘Lot’ or ‘Batch’.
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
A facial synthesis and antimicrobial activity of some pyrazole derivatives carrying indole.
Sarma KN, et al.
Journal of Chemistry, 7(3), 745-750 (2010)
Liu, Z.; et al.
Letters in Organic Chemistry, 7, 666-666 (2010)
Ube, H.; et al.
Tetrahedron Asymmetry, 21, 1203-1203 (2010)
Fei Yang et al.
Organic letters, 12(22), 5214-5217 (2010-10-23)
A novel cross dehydrogenative coupling (CDC) reaction of N,N-dimethylanilines with methyl ketones by cooperative copper and aminocatalysis has been developed, which leads to the formation of β-arylamino ketones in 42-73% yields. Moreover, the copper-catalyzed alkylation of free (NH) indoles with
Wen-Jie Lu et al.
European journal of medicinal chemistry, 64, 498-511 (2013-05-21)
This report describes the synthesis, and in vitro and in vivo antimalarial evaluations of certain ester-modified neocryptolepine (5-methyl-5H-indolo[2,3-b]quinoline) derivatives. The modifications were carried out by introducing ester groups at the C2 and/or C9 position on the neocryptolepine core and the
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service