All Photos(1)
About This Item
Linear Formula:
ClC6H3(F)CHO
CAS Number:
Molecular Weight:
158.56
MDL number:
UNSPSC Code:
12352100
PubChem Substance ID:
NACRES:
NA.22
Recommended Products
Quality Level
Assay
97%
form
solid
bp
118-120 °C/50 mmHg (lit.)
mp
60-63 °C (lit.)
functional group
aldehyde
chloro
fluoro
SMILES string
Fc1ccc(C=O)c(Cl)c1
InChI
1S/C7H4ClFO/c8-7-3-6(9)2-1-5(7)4-10/h1-4H
InChI key
KMQWNQKESAHDKD-UHFFFAOYSA-N
General description
2-Chloro-4-fluorobenzaldehyde is a halogen substituted benzaldehyde.
Application
2-Chloro-4-fluorobenzaldehyde may be used in the synthesis of substituted α-cyanocinnamic acid, via Knoevenagel condensation reaction. It may be used in the synthesis of 2-(2-benzimidazolyl)-3-(2-chloro-4-fluorophenyl)acrylonitrile.
Signal Word
Warning
Hazard Statements
Precautionary Statements
Hazard Classifications
Eye Irrit. 2 - Skin Irrit. 2 - STOT SE 3
Target Organs
Respiratory system
Storage Class Code
11 - Combustible Solids
WGK
WGK 3
Flash Point(F)
Not applicable
Flash Point(C)
Not applicable
Personal Protective Equipment
dust mask type N95 (US), Eyeshields, Gloves
Choose from one of the most recent versions:
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Biological activity and DNA binding studies of 2-substituted benzimidazo [1, 2-a] quinolines bearing different amino side chains.
Perin N, et al.
MedChemComm, 4(12), 1537-1550 (2013)
Thorsten W Jaskolla et al.
Proceedings of the National Academy of Sciences of the United States of America, 105(34), 12200-12205 (2008-08-30)
Matrix-assisted laser desorption ionization (MALDI) has become an enabling technology for the fields of protein mass spectrometry (MS) and proteomics. Despite its widespread use, for example, in protein identification via peptide mass fingerprinting, a comprehensive model for the generation of
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service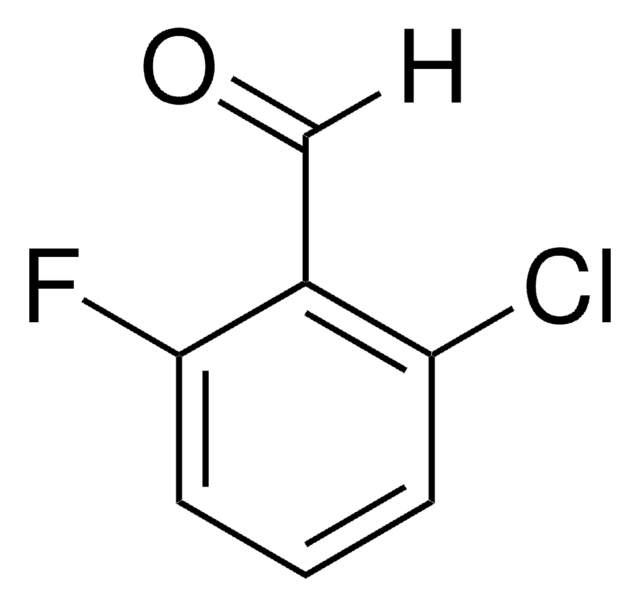
![Benzo[b]thiophene-2-carboxaldehyde 97%](/deepweb/assets/sigmaaldrich/product/structures/321/060/32405a4e-5720-4c6d-91cf-115c747270c4/640/32405a4e-5720-4c6d-91cf-115c747270c4.png)