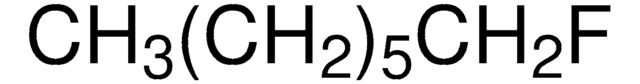All Photos(1)
About This Item
Linear Formula:
CH3(CH2)4F
CAS Number:
Molecular Weight:
90.14
EC Number:
MDL number:
UNSPSC Code:
12352100
PubChem Substance ID:
NACRES:
NA.22
Recommended Products
Assay
98%
form
liquid
refractive index
n20/D 1.36 (lit.)
bp
62-63 °C (lit.)
density
0.789 g/mL at 25 °C (lit.)
SMILES string
CCCCCF
InChI
1S/C5H11F/c1-2-3-4-5-6/h2-5H2,1H3
InChI key
OEPRBXUJOQLYID-UHFFFAOYSA-N
General description
Dual luminescence of 4-N,N-dimethylaminobenzonitrile has been observed in 1-fluoropentane.
Application
1-Fluoropentane has been used in the synthesis of 6-F-B10H13 and 6-Cl- B10H13 (halodecaboranes).
accessory
Product No.
Description
Pricing
Signal Word
Danger
Hazard Statements
Precautionary Statements
Hazard Classifications
Eye Irrit. 2 - Flam. Liq. 2 - Skin Irrit. 2 - STOT SE 3
Target Organs
Respiratory system
Storage Class Code
3 - Flammable liquids
WGK
WGK 3
Flash Point(F)
-9.4 °F - closed cup
Flash Point(C)
-23 °C - closed cup
Personal Protective Equipment
dust mask type N95 (US), Eyeshields, Gloves
Certificates of Analysis (COA)
Search for Certificates of Analysis (COA) by entering the products Lot/Batch Number. Lot and Batch Numbers can be found on a product’s label following the words ‘Lot’ or ‘Batch’.
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Customers Also Viewed
C F Nhachi et al.
Food and chemical toxicology : an international journal published for the British Industrial Biological Research Association, 26(8), 705-713 (1988-08-01)
Phenobarbitone pretreatment potentiated hepatocyte lesions in male rats 24 hr after treatment with 1-fluoropentane (3.5 mg/kg body weight) and 1-fluorohexane (0.17 mg/kg body weight). Serum levels of the enzymes ornithine carbamyltransferase, glutamic-pyruvic transaminase and gamma-glutamyltranspeptidase were significantly elevated by the
K R Harikumar et al.
Nature chemistry, 1(9), 716-721 (2010-12-03)
The controlled imprinting of surfaces with specified patterns is important in the development of nanoscale devices. Previously, such patterns were created using self-assembled physisorbed adsorbate molecules that can be stabilized on the surface by subsequent chemical bonding. Here we show
William C Ewing et al.
Inorganic chemistry, 47(19), 8580-8582 (2008-08-30)
The high-yield syntheses of 6-X-B 10H 13 [X = Cl (88%), Br (96%), I (84%)] resulted from the cage-opening reactions of the (NH 4 (+)) 2B 10H 10 (2-) salt with ionic-liquid-based superacidic hydrogen halides, while both the previously unknown
The role of the solvent in the dual luminescence of 4-N, N-dimethylaminobenzonitrile.
Suppan P.
Chemical Physics Letters, 128(2), 160-161 (1986)
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service