All Photos(1)
About This Item
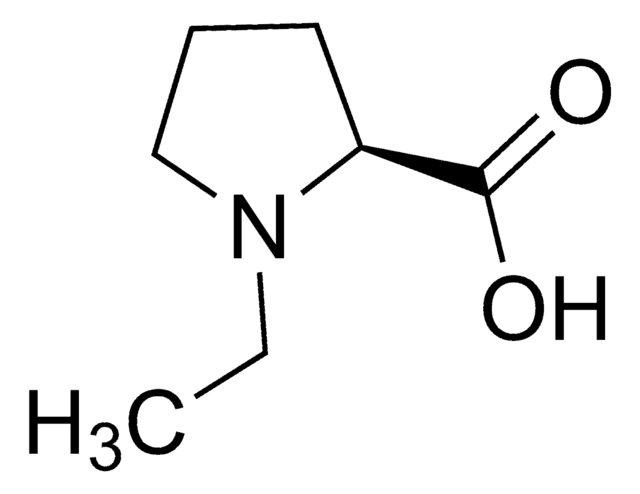
Empirical Formula (Hill Notation):
C6H11NO2
CAS Number:
Molecular Weight:
129.16
Beilstein:
4350211
MDL number:
UNSPSC Code:
12352209
PubChem Substance ID:
NACRES:
NA.22
Recommended Products
Assay
≥98.0% (TLC)
reaction suitability
reaction type: solution phase peptide synthesis
application(s)
peptide synthesis
SMILES string
C[C@]1(CCCN1)C(O)=O
InChI
1S/C6H11NO2/c1-6(5(8)9)3-2-4-7-6/h7H,2-4H2,1H3,(H,8,9)/t6-/m0/s1
InChI key
LWHHAVWYGIBIEU-LURJTMIESA-N
Related Categories
Storage Class Code
11 - Combustible Solids
WGK
WGK 3
Flash Point(F)
Not applicable
Flash Point(C)
Not applicable
Personal Protective Equipment
dust mask type N95 (US), Eyeshields, Gloves
Certificates of Analysis (COA)
Search for Certificates of Analysis (COA) by entering the products Lot/Batch Number. Lot and Batch Numbers can be found on a product’s label following the words ‘Lot’ or ‘Batch’.
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Alessandro Moretto et al.
Biopolymers, 89(5), 465-470 (2007-09-07)
Methylation at the C(alpha)-position of a Pro residue was expected to lock the preceding tertiary amide (omega) torsion angle of the resulting (alphaMe)Pro to the trans disposition and to restrict the phi,psi surface to the single region where the 3(10)/alpha-helices
S Thaisrivongs et al.
Journal of medicinal chemistry, 30(3), 536-541 (1987-03-01)
A structure-activity analysis of peptides containing backbone C alpha-methyl modification at the P4 site of the angiotensinogen sequence led to the discovery of potent renin inhibitors with apparent in vitro metabolic stability. Boc-alpha-MePro-Phe-His-Leu psi[CHOHCH2]Val-Ile-Amp dicitrate (Va) is a potent inhibitor
P W Baures et al.
The journal of peptide research : official journal of the American Peptide Society, 50(1), 1-13 (1997-07-01)
The effect of replacing one of the proline residues in either unsubstituted homochiral or heterochiral diproline segments with either a 2- or a 3-substituted prolyl residue on the allowed conformational of the diproline template has been examined. In heterochiral (L-D)
Matteo De Poli et al.
Chemistry (Weinheim an der Bergstrasse, Germany), 15(32), 8015-8025 (2009-07-07)
C(alpha)-methyl-L-proline, or L-(alphaMe)Pro, is probably the most conformationally constrained alpha-amino acid. In particular, its omega and phi torsion angles are restricted to about 180 and -60 degrees, respectively, and only three ranges of values are theoretically available for psi in
Guillem Revilla-López et al.
Biopolymers, 98(2), 98-110 (2011-09-08)
The structural consequences derived from the incorporation of either a methyl or a phenyl group at the α carbon of proline were recently investigated by quantum mechanical calculations (J Org Chem 2008, 73, 3418). In this work, the effect produced
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service