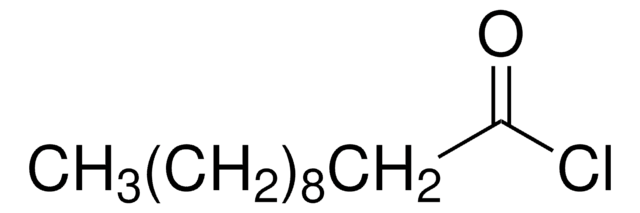171476
Undecanoic acid
98%
Synonym(s):
Hendecanoic acid
Sign Into View Organizational & Contract Pricing
All Photos(2)
About This Item
Linear Formula:
CH3(CH2)9COOH
CAS Number:
Molecular Weight:
186.29
Beilstein:
1759287
EC Number:
MDL number:
UNSPSC Code:
12352100
PubChem Substance ID:
NACRES:
NA.22
Recommended Products
Assay
98%
form
solid
bp
228 °C/160 mmHg (lit.)
248-250 °C (lit.)
mp
28-31 °C (lit.)
functional group
carboxylic acid
SMILES string
CCCCCCCCCCC(O)=O
InChI
1S/C11H22O2/c1-2-3-4-5-6-7-8-9-10-11(12)13/h2-10H2,1H3,(H,12,13)
InChI key
ZDPHROOEEOARMN-UHFFFAOYSA-N
Looking for similar products? Visit Product Comparison Guide
Related Categories
Application
Undecanoic acid was used in the formation of cycloamylose crystals.
Storage Class Code
11 - Combustible Solids
WGK
WGK 1
Flash Point(F)
>233.6 °F
Flash Point(C)
> 112 °C
Personal Protective Equipment
dust mask type N95 (US), Eyeshields, Gloves
Choose from one of the most recent versions:
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Customers Also Viewed
Rashanique D Quarels et al.
Beilstein journal of nanotechnology, 8, 1863-1877 (2017-10-20)
Visible-light irradiation of phthalimide esters in the presence of the photosensitizer [Ru(bpy)
Fernanda G Paião et al.
FEMS microbiology letters, 271(2), 180-186 (2007-04-12)
Suppressive subtractive hybridization was used to isolate transcripts specifically upregulated during Trichophyton rubrum exposure to acriflavin, fluconazole, griseofulvin, terbinafine or undecanoic acid. Macro-array dot-blot and sequencing of 132 clones, which correspond to genes differentially expressed after exposition of T. rubrum
Margarida M L M Vareiro et al.
Analytical biochemistry, 377(2), 243-250 (2008-04-03)
The development of a single-step, separation-free method for measurement of low concentrations of fatty acid using a surface plasmon resonance-enhanced fluorescence competition assay with a surface-bound antibody is described. The assay behavior was unexpectedly complex. A nonlinear coverage-dependent self-quenching of
Aisha Laguerre et al.
Acta crystallographica. Section F, Structural biology and crystallization communications, 67(Pt 2), 291-295 (2011-02-09)
Fatty-acid binding proteins (FABPs) are abundantly expressed proteins that bind a range of lipophilic molecules. They have been implicated in the import and intracellular distribution of their ligands and have been linked with metabolic and inflammatory responses in the cells
Raluca Voicu et al.
Langmuir : the ACS journal of surfaces and colloids, 20(26), 11713-11720 (2004-12-15)
This paper describes a simple strategy for DNA immobilization on chemically modified and patterned silicon surfaces. The photochemical modification of hydrogen-terminated Si(111) with undecylenic acid leads to the formation of an organic monolayer covalently attached to the surface through Si-C
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service