Preparation of Chitosan Microparticles by Spray Drying
Véronique Maquet, Sandrine Gautier
KitoZyme, Rue Haute Claire 4, 4040 Herstal, Belgium
Introduction
Microparticles with controlled size and morphology are of significant interest in the fields of drug delivery and biopharmaceuticals. The objective of this study was to assess the effect of processing parameters on the ability to control the size and distribution of chitosan based microparticles, targeting diameter range of 1-10 µm. The effect of encapsulating bovine serum albumin (BSA), a model protein, as well as sodium tripolyphosphate (TPP), a cross-linker, was studied.
Materials and Method
Chitosan with average viscosimetric molecular weight of 67,000 g/mole and degree of acetylation of 16 mole % was used in this study (Prod. No. 740063). Solutions comprised of different compositions were prepared in the presence or absence of BSA. In some formulations, sodium tripolyphosphate (TPP, Prod. No. 238503) was used as a cross-linking agent. Acetic acid (HAc) at a concentration of 1 volume % was used as the solvent for chitosan (dissolution overnight, room temperature).
The compositions of the formulations evaluated in this study are reported in Table 1
Microparticles were prepared using a mini spray dryer (B-290) from BUCHI. The atomization parameters are reported in Table 2.
Parameters detailed in P1 were used for formulations F1 to F4, while parameters in P2 were tested on formulations F1 and F5.
The microparticles formed using the different formulations have been imaged by scanning electron microscopy (SEM) using a SEM JSL-840A. The particle size distribution was analyzed using laser diffractometry on a Mastersizer 2000 equipped with a Hydrosizer 2000 module (Malvern Instruments, UK).
Results
SEM images at two magnifications show that chitosan microparticles prepared from formulation 1 (without BSA or TPP) using parameter P1 are spherical with a slightly wrinkled surface (Figure 1).

Figure 1. SEM images of chitosan microparticles prepared from formulation F1 using parameters P1 at a) 2,000 x and b) 5,000 x magnification.
Formulation F2 was used to prepare chitosan-TPP microparticles under parameter P1in order to observe the effect of TPP cross-linker. SEM analysis indicates that addition of TPP does not alter the appearance or size of the resulting microparticles, as seen in Figure 2.

Figure 2. SEM images of chitosan-TPP microparticles prepared from formulation F2 using parameters P1 at a) 2,000 X and b) 5,000 x magnification.
The effect of loading chitosan microparticles with a model drug was investigated in formulation F3 with parameter P1, in the absence of TPP cross-linker. Figure 3 shows that the addition of BSA did not affect the appearance or size.

Figure 3. SEM images of chitosan-BSA microparticles prepared from formulation F3 using parameters P1 at a) 2,000 x and b) 5,000 x magnification.
Incorporation of BSA into the chitosan microparticle in the presence of TPP was studied in formulation F4 using parameter P1. In the presence of both TPP and BSA, microparticles appear less spherical (Figure 4).

Figure 4. SEM images of chitosan-BSA-TPP microparticles prepared from formulation F4 using parameters P1 at a) 2,000 x and b) 5,000 x magnification.
Formulation F5 (which is one tenth the chitosan concentration found in formulation F1) and using parameters P2, the chitosan particles appear much less spherical and tend to form aggregates composed of smaller particles (Figure 5).
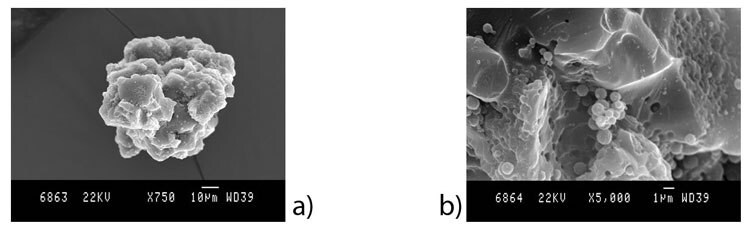
Figure 5. SEM images of chitosan microparticles prepared in formulation F5 using parameters P2 at a) 2,000 x and b) 5,000 x magnifaction.
As reported in Table 3, the microparticle size distribution is similar in formulations F1-F3, with an average particle diameter, D(0.5) between 3.2-3.5 µm, 63 – 65 % of particles with size in the range 0.5 – 5 µm, 23-25 % of particles with size in the range 5-10 µm. Addition of both BSA and TPP slightly modifies the particle size distribution. The volume of particles in the range 0.5-5 µm slightly decrease in comparison to formulation F1-F3 while particles of 5-10 µm are more represented, leading to D(0.5) of 4.3 µm. When using parameters P2, the formulation F1 leads to smaller particles than those prepared using parameters P1.
Conclusion
These experiments show the feasibility of preparation of chitosan microparticles in different environments and under different conditions. Cross linking chitosan with TPP or encapsulation of BSA does not modify the morphology or size distribution of the chitosan particles, which had an average diameter of 3.2 – 3.5 µm. When both BSA and TPP were incorporated, the particle size increased to 4.3 µm, and the particle size distribution slightly widened.
When a more dilute solution of chitosan (0.1 % chitosan vs 1 % chitosan) was used, microparticles were still generated but were significantly more polydisperse. These particles appeared to be larger and contain agglomerations of smaller particles.
Another important factor affecting the properties of the particles formed was the spray drying parameters. Processing conditions for spray drying influenced particle size and distribution. Using identical solutions (1 wt.% chitosan solution in 1 v.% acetic acid), two different spray-drying parameters were used to illustrate the ability to generate particles with either an average diameter of 3.5 µm (in the case of F1/P1) or 2.5 µm (in the case of F1/P2).
Pour continuer à lire, veuillez vous connecter à votre compte ou en créer un.
Vous n'avez pas de compte ?