1138008
USP
Clindamycin phosphate
United States Pharmacopeia (USP) Reference Standard
Synonyme(s) :
Clindamycin 2-phosphate, Clindamycin 2-dihydrogen phosphate
About This Item
Produits recommandés
Qualité
pharmaceutical primary standard
Famille d'API
clindamycin
Fabricant/nom de marque
USP
Application(s)
pharmaceutical (small molecule)
Format
neat
Température de stockage
−20°C
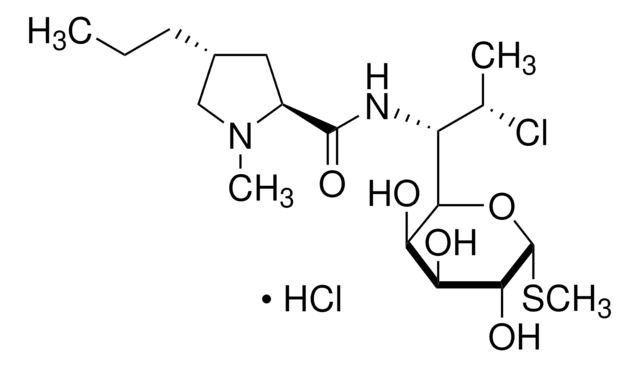
Chaîne SMILES
CCC[C@@H]1C[C@H](N(C)C1)C(=O)NC([C@H](C)Cl)C2O[C@H](SC)[C@H](OP(O)(O)=O)[C@@H](O)[C@H]2O
InChI
1S/C18H34ClN2O8PS/c1-5-6-10-7-11(21(3)8-10)17(24)20-12(9(2)19)15-13(22)14(23)16(18(28-15)31-4)29-30(25,26)27/h9-16,18,22-23H,5-8H2,1-4H3,(H,20,24)(H2,25,26,27)/t9-,10+,11-,12?,13+,14-,15?,16+,18+/m0/s1
Clé InChI
UFUVLHLTWXBHGZ-MWBQRTRKSA-N
Vous recherchez des produits similaires ? Visite Guide de comparaison des produits
Description générale
Application
- Clindamycin Phosphate Gel
- Clindamycin Phosphate Vaginal Cream
- Clindamycin Phosphate Topical Suspension
- Clindamycin Phosphate Vaginal Inserts
- Clindamycin Phosphate Topical Solution
- Clindamycin Injection
Actions biochimiques/physiologiques
Mode of Action: Inhibits protein synthesis in bacterial by binding the 50s ribosomal subunit.
Autres remarques
Remarque sur l'analyse
Produit(s) apparenté(s)
Mention d'avertissement
Warning
Mentions de danger
Conseils de prudence
Classification des risques
Acute Tox. 4 Oral - Eye Irrit. 2 - Lact. - Skin Sens. 1
Code de la classe de stockage
11 - Combustible Solids
Classe de danger pour l'eau (WGK)
WGK 3
Point d'éclair (°F)
Not applicable
Point d'éclair (°C)
Not applicable
Certificats d'analyse (COA)
Recherchez un Certificats d'analyse (COA) en saisissant le numéro de lot du produit. Les numéros de lot figurent sur l'étiquette du produit après les mots "Lot" ou "Batch".
Déjà en possession de ce produit ?
Retrouvez la documentation relative aux produits que vous avez récemment achetés dans la Bibliothèque de documents.
Notre équipe de scientifiques dispose d'une expérience dans tous les secteurs de la recherche, notamment en sciences de la vie, science des matériaux, synthèse chimique, chromatographie, analyse et dans de nombreux autres domaines..
Contacter notre Service technique