G9793
N-Glycolylneuraminic acid
≥95% (HPLC), semisynthetic
Synonyme(s) :
Neu5Glc, NeuNGl
About This Item
Produits recommandés
Source biologique
semisynthetic
Niveau de qualité
Pureté
≥95% (HPLC)
Forme
powder
Technique(s)
LC/MS: suitable
Impuretés
water (Karl Fischer)
Couleur
white
Solubilité
water: soluble 20 mg/mL
Adéquation
suitable for LC-MS
Application(s)
metabolomics
Température de stockage
−20°C
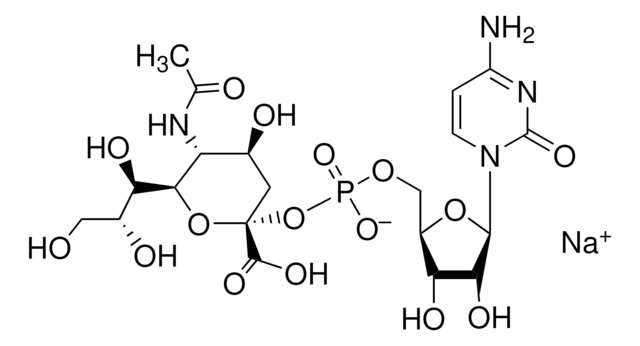
Chaîne SMILES
[H][C@]1(O[C@@](O)(C[C@H](O)[C@H]1NC(=O)CO)C(O)=O)[C@H](O)[C@H](O)CO
InChI
1S/C11H19NO10/c13-2-5(16)8(18)9-7(12-6(17)3-14)4(15)1-11(21,22-9)10(19)20/h4-5,7-9,13-16,18,21H,1-3H2,(H,12,17)(H,19,20)/t4-,5+,7+,8+,9+,11-/m0/s1
Clé InChI
FDJKUWYYUZCUJX-AJKRCSPLSA-N
Vous recherchez des produits similaires ? Visite Guide de comparaison des produits
Description générale
In humans, the absence of endogenous production results from a gene mutation affecting CMP-Neu5Ac hydroxylase, the enzyme responsible for converting N-acetylneuraminic acid into Neu5Gc. However, Neu5Gc can accumulate in human cells through external ingestion from dietary sources like red meat and dairy products. N-Glycolylneuraminic acid is a versatile compound that finds application in cell biology, metabolomics and biochemical research
Application
- as a sugar in microtiter biofilm methodologic approach for the enhancement of biofilm formation
- as a standard for the determination of sialic acids in the nervous system of silkworm and to find the variations of sialic acids among different developmental stages.
- as a standard in the high-performance liquid chromatography (HPLC) analyses to detect the molecular species of sialic acid (Sia) species using 1,2-diamino-4,5-methylenedioxy-benzene (DMB) as a fluorogenic compound
Actions biochimiques/physiologiques
Caractéristiques et avantages
- Ideal for Metabolomics, Biochemical and Cell Biology research
- Versatile and adaptable for wide variety of laboratory and research applications
Autres remarques
Produit comparable
Code de la classe de stockage
11 - Combustible Solids
Classe de danger pour l'eau (WGK)
WGK 3
Point d'éclair (°F)
Not applicable
Point d'éclair (°C)
Not applicable
Équipement de protection individuelle
Eyeshields, Gloves, type N95 (US)
Certificats d'analyse (COA)
Recherchez un Certificats d'analyse (COA) en saisissant le numéro de lot du produit. Les numéros de lot figurent sur l'étiquette du produit après les mots "Lot" ou "Batch".
Déjà en possession de ce produit ?
Retrouvez la documentation relative aux produits que vous avez récemment achetés dans la Bibliothèque de documents.
Les clients ont également consulté
Notre équipe de scientifiques dispose d'une expérience dans tous les secteurs de la recherche, notamment en sciences de la vie, science des matériaux, synthèse chimique, chromatographie, analyse et dans de nombreux autres domaines..
Contacter notre Service technique