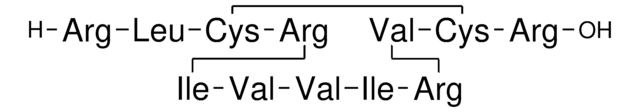C5241
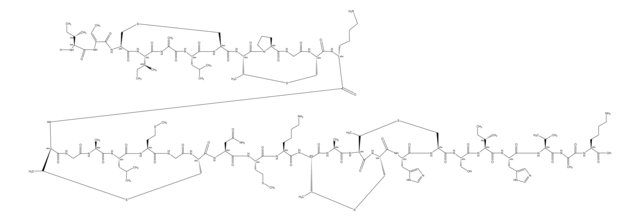
Cinnamycin
from Streptomyces cinnamoneus, ≥95% (HPLC)
Synonyme(s) :
Lanthiopeptin, NSC-71936, Ro 09-0198
About This Item
Produits recommandés
Source biologique
Streptomyces cinnamoneus
Niveau de qualité
Pureté
≥95% (HPLC)
Forme
solid
Solubilité
DMSO: 10 mg/mL
acetonitrile: water (1:1): 5 mg/mL (requires heating)
Spectre d'activité de l'antibiotique
fungi
Mode d’action
cell membrane | interferes
Température de stockage
2-8°C
InChI
1S/C89H125N25O25S3/c1-43(2)66-84(133)109-59-41-140-40-58-79(128)108-60-42-142-45(4)68(86(135)105-55(75(124)110-66)34-48-22-12-7-13-23-48)111-76(125)54(33-47-20-10-6-11-21-47)104-82(131)61-26-17-31-114(61)65(118)38-98-72(121)53(32-46-18-8-5-9-19-46)103-78(127)57(106-80(60)129)36-95-29-15-14-24-52(87(136)137)102-85(134)67(112-77(126)56(35-63(92)116)99-64(117)37-97-83(132)69(113-81(59)130)70(119)88(138)139)44(3)141-39-49(90)71(120)100-50(25-16-30-96-89(93)94)73(122)101-51(74(123)107-58)27-28-62(91)115/h5-13,18-23,43-45,49-61,66-70,95,119H,14-17,24-42,90H2,1-4H3,(H2,91,115)(H2,92,116)(H,97,132)(H,98,121)(H,99,117)(H,100,120)(H,101,122)(H,102,134)(H,103,127)(H,104,131)(H,105,135)(H,106,129)(H,107,123)(H,108,128)(H,109,133)(H,110,124)(H,111,125)(H,112,126)(H,113,130)(H,136,137)(H,138,139)(H4,93,94,96)/t44-,45?,49+,50+,51+,52+,53+,54+,55+,56+,57+,58+,59+,60+,61+,66+,67?,68+,69+,70-/m1/s1
Clé InChI
QJDWKBINWOWJNZ-IDGBIKHQSA-N
Catégories apparentées
Amino Acid Sequence
Description générale
Application
Actions biochimiques/physiologiques
Cinnamycin, like other lantibiotics, was also reported to inhibit phospholipase A2 (PLA2). It was suggested as an alternative treatment for atherosclerosis through its ability to inhibit PLA2 by binding to its substrate PE. Moreover, Cinnamycin was found to inhibit Herpes simplex virus (HSV-1) activity.
Code de la classe de stockage
11 - Combustible Solids
Classe de danger pour l'eau (WGK)
WGK 3
Point d'éclair (°F)
Not applicable
Point d'éclair (°C)
Not applicable
Équipement de protection individuelle
Eyeshields, Gloves, type N95 (US)
Certificats d'analyse (COA)
Recherchez un Certificats d'analyse (COA) en saisissant le numéro de lot du produit. Les numéros de lot figurent sur l'étiquette du produit après les mots "Lot" ou "Batch".
Déjà en possession de ce produit ?
Retrouvez la documentation relative aux produits que vous avez récemment achetés dans la Bibliothèque de documents.
Les clients ont également consulté
Articles
Ribosomally synthesized antimicrobial peptides are a promising focus in antibiotic research amidst bacterial resistance and emerging infectious diseases.
Ribosomally synthesized antimicrobial peptides are a promising focus in antibiotic research amidst bacterial resistance and emerging infectious diseases.
Ribosomally synthesized antimicrobial peptides are a promising focus in antibiotic research amidst bacterial resistance and emerging infectious diseases.
Ribosomally synthesized antimicrobial peptides are a promising focus in antibiotic research amidst bacterial resistance and emerging infectious diseases.
Notre équipe de scientifiques dispose d'une expérience dans tous les secteurs de la recherche, notamment en sciences de la vie, science des matériaux, synthèse chimique, chromatographie, analyse et dans de nombreux autres domaines..
Contacter notre Service technique