PHR1039
Erythromycin
Pharmaceutical Secondary Standard; Certified Reference Material
About This Item
Produits recommandés
Qualité
certified reference material
pharmaceutical secondary standard
Niveau de qualité
Agence
traceable to BP 794
traceable to Ph. Eur. E1305000
traceable to USP 1242000
Famille d'API
erythromycin
CofA (certificat d'analyse)
current certificate can be downloaded
Technique(s)
HPLC: suitable
gas chromatography (GC): suitable
Application(s)
pharmaceutical (small molecule)
Format
neat
Température de stockage
-10 to -25°C
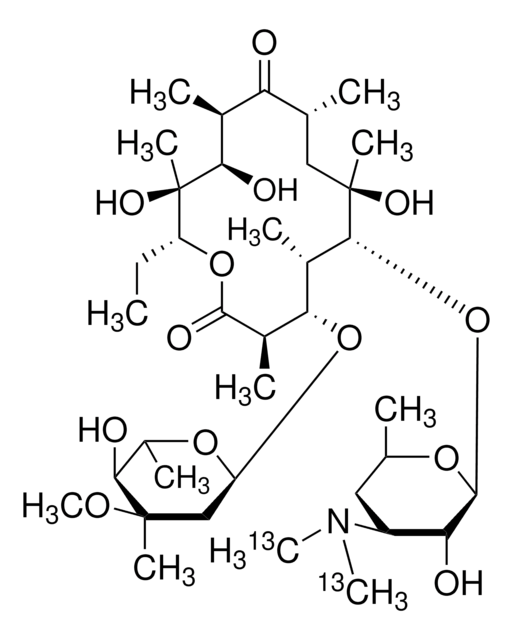
Chaîne SMILES
CC[C@H]1OC(=O)[C@H](C)[C@@H](O[C@H]2C[C@@](C)(OC)[C@@H](O)[C@H](C)O2)[C@H](C)[C@@H](O[C@@H]3O[C@H](C)C[C@@H]([C@H]3O)N(C)C)[C@](C)(O)C[C@@H](C)C(=O)[C@H](C)[C@@H](O)[C@]1(C)O
InChI
1S/C37H67NO13/c1-14-25-37(10,45)30(41)20(4)27(39)18(2)16-35(8,44)32(51-34-28(40)24(38(11)12)15-19(3)47-34)21(5)29(22(6)33(43)49-25)50-26-17-36(9,46-13)31(42)23(7)48-26/h18-26,28-32,34,40-42,44-45H,14-17H2,1-13H3/t18-,19-,20+,21+,22-,23+,24+,25-,26+,28-,29+,30-,31+,32-,34+,35-,36-,37-/m1/s1
Clé InChI
ULGZDMOVFRHVEP-RWJQBGPGSA-N
Vous recherchez des produits similaires ? Visite Guide de comparaison des produits
Description générale
Erythromycin is a complex macrolide antibiotic drug that exhibits a bacteriostatic activity. It is generally employed in human and veterinary medicines owing to its potential activity against gram positive and a few gram negative strains.
Application
Actions biochimiques/physiologiques
Antimicrobial Spectrum: This product acts against both gram-negative and gram-positive bacteria.
Attention
Notes préparatoires
Note de bas de page
Informations légales
These secondary standards offer multi-traceability to the USP, EP (PhEur) and BP primary standards, where they are available. This product was designed, produced and verified for accuracy and stability in accordance with ISO/IEC 17025:2005 (AClass Cert AT-1467), ISO GUIDES 34:2009 (AClass Cert AR-1470).
Produit(s) apparenté(s)
Code de la classe de stockage
11 - Combustible Solids
Classe de danger pour l'eau (WGK)
WGK 3
Point d'éclair (°F)
Not applicable
Point d'éclair (°C)
Not applicable
Faites votre choix parmi les versions les plus récentes :
Certificats d'analyse (COA)
Vous ne trouvez pas la bonne version ?
Si vous avez besoin d'une version particulière, vous pouvez rechercher un certificat spécifique par le numéro de lot.
Déjà en possession de ce produit ?
Retrouvez la documentation relative aux produits que vous avez récemment achetés dans la Bibliothèque de documents.
Les clients ont également consulté
Articles
Comparative analysis of Supel™ BioSPME 96-Pin device with a rapid equilibrium dialysis technique for accuracy of measured values, sample cleanliness, and workflow time in drug protein binding.
Comparative analysis of Supel™ BioSPME 96-Pin device with a rapid equilibrium dialysis technique for accuracy of measured values, sample cleanliness, and workflow time in drug protein binding.
Comparative analysis of Supel™ BioSPME 96-Pin device with a rapid equilibrium dialysis technique for accuracy of measured values, sample cleanliness, and workflow time in drug protein binding.
Comparative analysis of Supel™ BioSPME 96-Pin device with a rapid equilibrium dialysis technique for accuracy of measured values, sample cleanliness, and workflow time in drug protein binding.
Notre équipe de scientifiques dispose d'une expérience dans tous les secteurs de la recherche, notamment en sciences de la vie, science des matériaux, synthèse chimique, chromatographie, analyse et dans de nombreux autres domaines..
Contacter notre Service technique





![(1S,6R)-6-HYDROXY-5-METHYLENE-1-[(1S)-1,2,3-TRIHYDROXY-2-METHYLPROPYL]-2-OXA-7,9-DIAZABICYCLO[4.2.2]DECANE-8,10-DIONE AldrichCPR](/deepweb/assets/sigmaaldrich/product/structures/271/983/0a99c1ad-6200-4f41-b31f-bd929e66599d/640/0a99c1ad-6200-4f41-b31f-bd929e66599d.png)