MTOX1000CC24
Caco-2 Engineered Control Cells
Human male colorectal tissue, adenocarcinoma
About This Item
Produits recommandés
product name
Caco-2 Engineered Control Cells, one assay ready, 24-well plate
Source biologique
human male colorectal tissue (Source Disease: adenocarcinoma)
Forme
liquid
Description générale
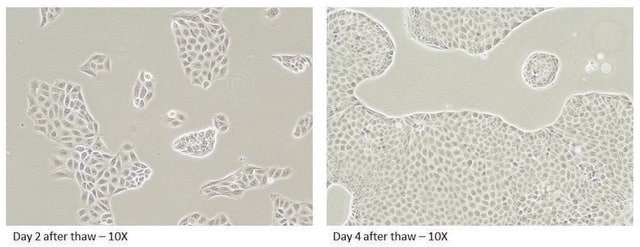
The three week production lead time begins on the Monday following a purchase, in the third week the plates are shipped on Tuesday for receipt on Wednesday or Thursday. As a biologic product that is shipped at room temperature the cells must be processed immediately upon receipt.
A minimum of 4 assay ready plates is required to purchase. Please allow for 1 week after ordering, for the plates to be seeded. Plates will be shipped to the customer on day 16 of the 21-day culturing process.
Application
Transporter Function in Caco-2 Cells with Targeted P-Glycoprotein, MRP2 and BCRP Gene Knockout Using Zinc Finger Nucleases
Comparison of Function and Relative Transporter Protein Concentrations in Caco-2 Cells with Single and Double Knockouts of the ABCB1, ABCG2, and ABCC2 Genes
Caco-2 Transporter Knockout Cell Based Assays
Caractéristiques et avantages
- The 24-well Transwell format enables the these cells to be included in standard drug transporter protocols
- Human assay with no interference from animal inhibitors
- The 21-day cell culture period is reduced to 6-days for customers, saving valuable resources
Qualité
Informations légales
Produit(s) apparenté(s)
Code de la classe de stockage
10 - Combustible liquids
Classe de danger pour l'eau (WGK)
WGK 3
Point d'éclair (°F)
Not applicable
Point d'éclair (°C)
Not applicable
Faites votre choix parmi les versions les plus récentes :
Certificats d'analyse (COA)
Vous ne trouvez pas la bonne version ?
Si vous avez besoin d'une version particulière, vous pouvez rechercher un certificat spécifique par le numéro de lot.
Déjà en possession de ce produit ?
Retrouvez la documentation relative aux produits que vous avez récemment achetés dans la Bibliothèque de documents.
Articles
We presents an article on The Role of Intestinal Efflux Transporters In Drug Absorption.
We presents an article on The Role of Intestinal Efflux Transporters In Drug Absorption.
We presents an article on The Role of Intestinal Efflux Transporters In Drug Absorption.
We presents an article on The Role of Intestinal Efflux Transporters In Drug Absorption.
Notre équipe de scientifiques dispose d'une expérience dans tous les secteurs de la recherche, notamment en sciences de la vie, science des matériaux, synthèse chimique, chromatographie, analyse et dans de nombreux autres domaines..
Contacter notre Service technique