45400
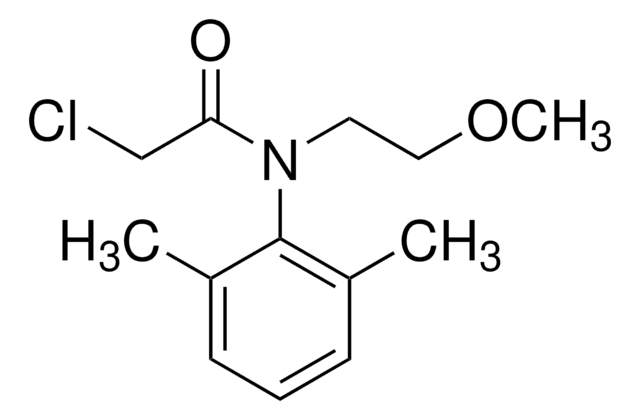
Chlortoluron
PESTANAL®, analytical standard
Synonyme(s) :
3-(3-Chloro-4-methyl)-1,1-dimethylurea
About This Item
Produits recommandés
Qualité
analytical standard
Niveau de qualité
Gamme de produits
PESTANAL®
Durée de conservation
limited shelf life, expiry date on the label
Technique(s)
HPLC: suitable
gas chromatography (GC): suitable
Application(s)
agriculture
cleaning products
cosmetics
environmental
food and beverages
personal care
Format
neat
Chaîne SMILES
CN(C)C(=O)Nc1ccc(C)c(Cl)c1
InChI
1S/C10H13ClN2O/c1-7-4-5-8(6-9(7)11)12-10(14)13(2)3/h4-6H,1-3H3,(H,12,14)
Clé InChI
JXCGFZXSOMJFOA-UHFFFAOYSA-N
Vous recherchez des produits similaires ? Visite Guide de comparaison des produits
Application
Produits recommandés
Informations légales
Mention d'avertissement
Warning
Mentions de danger
Conseils de prudence
Classification des risques
Aquatic Acute 1 - Aquatic Chronic 1 - Carc. 2 - Repr. 2
Code de la classe de stockage
11 - Combustible Solids
Classe de danger pour l'eau (WGK)
WGK 3
Point d'éclair (°F)
Not applicable
Point d'éclair (°C)
Not applicable
Faites votre choix parmi les versions les plus récentes :
Déjà en possession de ce produit ?
Retrouvez la documentation relative aux produits que vous avez récemment achetés dans la Bibliothèque de documents.
Notre équipe de scientifiques dispose d'une expérience dans tous les secteurs de la recherche, notamment en sciences de la vie, science des matériaux, synthèse chimique, chromatographie, analyse et dans de nombreux autres domaines..
Contacter notre Service technique