00180585
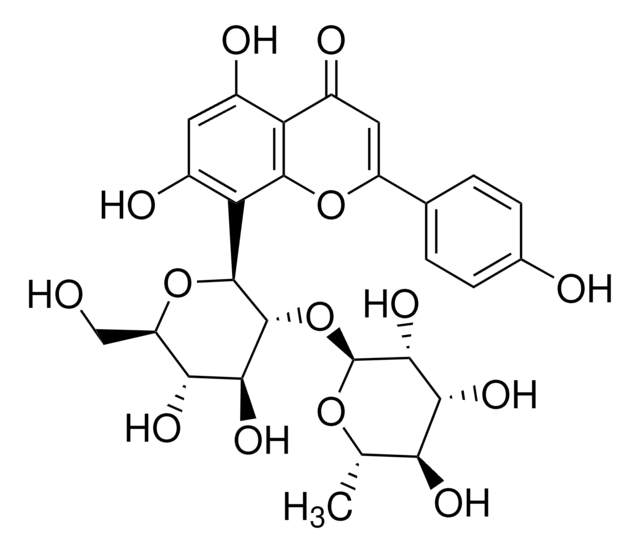
Hyperoside
primary reference standard
Synonyme(s) :
Quercetin 3-D-galactoside, 3,3′,4′,5,7-Pentahydroxyflavone 3-D-galactoside, Hyperin, Hyperoside
About This Item
Produits recommandés
Qualité
primary reference standard
Durée de conservation
limited shelf life, expiry date on the label
Fabricant/nom de marque
HWI
Technique(s)
HPLC: suitable
gas chromatography (GC): suitable
Chaîne SMILES
OC[C@H]1O[C@@H](OC2=C(Oc3cc(O)cc(O)c3C2=O)c4ccc(O)c(O)c4)[C@H](O)[C@@H](O)[C@H]1O
InChI
1S/C21H20O12/c22-6-13-15(27)17(29)18(30)21(32-13)33-20-16(28)14-11(26)4-8(23)5-12(14)31-19(20)7-1-2-9(24)10(25)3-7/h1-5,13,15,17-18,21-27,29-30H,6H2/t13-,15+,17+,18-,21+/m1/s1
Clé InChI
OVSQVDMCBVZWGM-DTGCRPNFSA-N
Vous recherchez des produits similaires ? Visite Guide de comparaison des produits
Description générale
Exact content measured by quantitative NMR can be found on the certificate.
Application
It can also be used as follows:
- Development of an ultra performance liquid chromatography-mass spectrometric method to determine hyperoside in rat plasma samples following sample treatment by protein precipitation and liquid-liquid extraction
- Multi-residue analysis of hyperoside, isoquercitrin, and eleutheroside E in botanical samples of Apocynum venetum L. and Eleutherococcus senticosus used in traditional Chinese medicine by HPLC combined with diode array detection (DAD)
- Ionic liquid vacuum microwave-assisted extraction (ILVMAE) of rutin, hyperoside, and hesperidin from Sorbus tianschanica leaf samples for their subsequent quantification by HPLC
- Simultaneous detection and quantification of vitexin, rutin, hyperoside, and quercetin in hawthorn flower and leaf extract samples using high-performance liquid chromatography combined with spectrophotometric detection (HPLC-UV)
Actions biochimiques/physiologiques
Autres remarques
Mention d'avertissement
Warning
Mentions de danger
Conseils de prudence
Classification des risques
Acute Tox. 4 Oral
Code de la classe de stockage
11 - Combustible Solids
Classe de danger pour l'eau (WGK)
WGK 3
Point d'éclair (°F)
Not applicable
Point d'éclair (°C)
Not applicable
Faites votre choix parmi les versions les plus récentes :
Certificats d'analyse (COA)
Vous ne trouvez pas la bonne version ?
Si vous avez besoin d'une version particulière, vous pouvez rechercher un certificat spécifique par le numéro de lot.
Déjà en possession de ce produit ?
Retrouvez la documentation relative aux produits que vous avez récemment achetés dans la Bibliothèque de documents.
Les clients ont également consulté
Protocoles
HPTLC fingerprinting enables rapid identification of passion flower with related reference materials.
HPTLC fingerprinting enables rapid identification of passion flower with related reference materials.
HPTLC fingerprinting enables rapid identification of passion flower with related reference materials.
HPTLC fingerprinting enables rapid identification of passion flower with related reference materials.
Notre équipe de scientifiques dispose d'une expérience dans tous les secteurs de la recherche, notamment en sciences de la vie, science des matériaux, synthèse chimique, chromatographie, analyse et dans de nombreux autres domaines..
Contacter notre Service technique