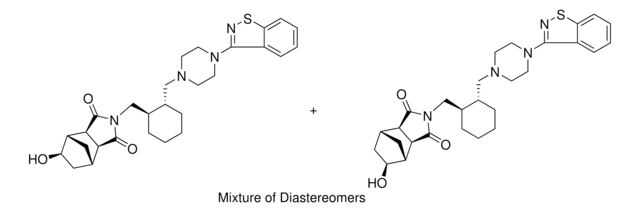
L-030
Lurasidone hydrochloride solution
1.0 mg/mL in methanol (as free base), ampule of 1 mL, certified reference material, Cerilliant®
About This Item
Produits recommandés
Qualité
certified reference material
Niveau de qualité
Forme
liquid
Caractéristiques
Snap-N-Spike®/Snap-N-Shoot®
Conditionnement
ampule of 1 mL
Fabricant/nom de marque
Cerilliant®
Concentration
1.0 mg/mL in methanol (as free base)
Technique(s)
gas chromatography (GC): suitable
liquid chromatography (LC): suitable
Application(s)
clinical testing
Format
single component solution
Température de stockage
−20°C
Chaîne SMILES
Cl.O=C1[C@@H]2[C@H]3CC[C@H](C3)[C@@H]2C(=O)N1C[C@@H]4CCCC[C@H]4CN5CCN(CC5)c6nsc7ccccc67
InChI
1S/C28H36N4O2S.ClH/c33-27-24-18-9-10-19(15-18)25(24)28(34)32(27)17-21-6-2-1-5-20(21)16-30-11-13-31(14-12-30)26-22-7-3-4-8-23(22)35-29-26;/h3-4,7-8,18-21,24-25H,1-2,5-6,9-17H2;1H/t18-,19+,20-,21-,24+,25-;/m0./s1
Clé InChI
NEKCRUIRPWNMLK-SCIYSFAVSA-N
Informations sur le gène
human ... DRD2(1813) , HTR2A(3356)
Vous recherchez des produits similaires ? Visite Guide de comparaison des produits
Description générale
Application
- LurasiDonein Bipolar Depression Research: A study explored the pharmacodynamic properties of lurasidone, hypothesizing its efficacy in acute bipolar depression. This research provides a deep dive into the mechanistic actions of lurasidone, enhancing understanding in neuropharmacological studies and aiding in the development of more effective treatments for bipolar disorder (Fountoulakis et al., 2024).
- Quantification of LurasiDonein Clinical Samples: Development and validation of a liquid chromatography-tandem mass spectrometry method for quantifying lurasiDonein dried blood spot samples was reported. This method facilitates easier and less invasive monitoring of lurasiDonelevels in patients, crucial for effective pharmacological research and ensuring therapeutic efficacy in treatment regimes (Rajadhyaksha and Londhe, 2023).
- Novel Methodologies in Clinical Trials: Research introduced a novel method for deriving adverse event prevalence in randomized controlled trials, which could potentially improve the understanding of the benefit-risk ratio of drugs including lurasidone. This approach is particularly relevant for drug labels and regulatory submissions, ensuring safer and more effective clinical outcomes (Piacentino et al., 2024).
- Pharmacological Properties of Lurasidone: A study investigated how lurasiDoneblocks the voltage-gated potassium channels of coronary arterial smooth muscle cells, offering insights into its broader pharmacological impacts. This research is vital for assessing potential cardiovascular side effects and optimizing dosing strategies to mitigate risks in patients treated with lurasiDone(Zhuang et al., 2023).
Informations légales
Mention d'avertissement
Danger
Mentions de danger
Conseils de prudence
Classification des risques
Acute Tox. 3 Dermal - Acute Tox. 3 Inhalation - Acute Tox. 3 Oral - Flam. Liq. 2 - STOT SE 1
Organes cibles
Eyes
Code de la classe de stockage
3 - Flammable liquids
Classe de danger pour l'eau (WGK)
WGK 1
Point d'éclair (°F)
49.5 °F - closed cup
Point d'éclair (°C)
9.7 °C - closed cup
Certificats d'analyse (COA)
Recherchez un Certificats d'analyse (COA) en saisissant le numéro de lot du produit. Les numéros de lot figurent sur l'étiquette du produit après les mots "Lot" ou "Batch".
Déjà en possession de ce produit ?
Retrouvez la documentation relative aux produits que vous avez récemment achetés dans la Bibliothèque de documents.
Notre équipe de scientifiques dispose d'une expérience dans tous les secteurs de la recherche, notamment en sciences de la vie, science des matériaux, synthèse chimique, chromatographie, analyse et dans de nombreux autres domaines..
Contacter notre Service technique