830865P
Avanti
18:0 PA
Avanti Research™ - A Croda Brand
Synonyme(s) :
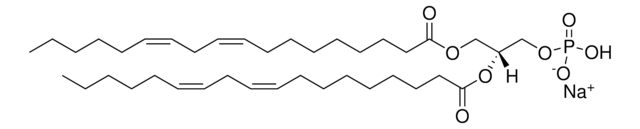
1,2-dioctadecanoyl-sn-glycero-3-phosphate (sodium salt); DSPA; PA(18:0/18:0)
About This Item
Produits recommandés
Description
1,2-distearoyl-sn-glycero-3-phosphate (sodium salt)
Pureté
>99% (TLC)
Forme
powder
Conditionnement
pkg of 1 × 25 mg (830865P-25mg)
pkg of 1 × 500 mg (830865P-500mg)
Fabricant/nom de marque
Avanti Research™ - A Croda Brand
Type de lipide
cardiolipins
phospholipids
Conditions d'expédition
dry ice
Température de stockage
−20°C
InChI
1S/C39H77O8P.Na/c1-3-5-7-9-11-13-15-17-19-21-23-25-27-29-31-33-38(40)45-35-37(36-46-48(42,43)44)47-39(41)34-32-30-28-26-24-22-20-18-16-14-12-10-8-6-4-2;/h37H,3-36H2,1-2H3,(H2,42,43,44);/q;+1/p-1/t37-;/m1./s1
Clé InChI
ALPWRKFXEOAUDR-GKEJWYBXSA-M
Description générale
Application
- as a component in the bilayer to study its vesiculation ability on different coat protein I (COPI) fission factor
- to study its effects on intracellular Ca2+ concentration ([Ca2+]i) in C6 rat glioma and L2071 mouse fibroblast cells
- to study its effects on phosphorylation of cytochrome b(558) alpha chain (p22phox)
Actions biochimiques/physiologiques
Conditionnement
Informations légales
Souvent commandé avec ce produit
Code de la classe de stockage
13 - Non Combustible Solids
Classe de danger pour l'eau (WGK)
WGK 3
Point d'éclair (°F)
Not applicable
Point d'éclair (°C)
Not applicable
Certificats d'analyse (COA)
Recherchez un Certificats d'analyse (COA) en saisissant le numéro de lot du produit. Les numéros de lot figurent sur l'étiquette du produit après les mots "Lot" ou "Batch".
Déjà en possession de ce produit ?
Retrouvez la documentation relative aux produits que vous avez récemment achetés dans la Bibliothèque de documents.
Notre équipe de scientifiques dispose d'une expérience dans tous les secteurs de la recherche, notamment en sciences de la vie, science des matériaux, synthèse chimique, chromatographie, analyse et dans de nombreux autres domaines..
Contacter notre Service technique