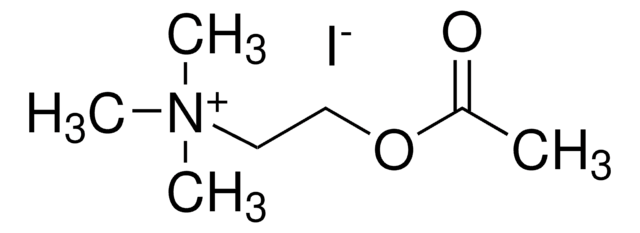
B3253
Butyrylthiocholine iodide
≥98%
Synonyme(s) :
(2-Mercaptoethyl)trimethylammonium iodide butyrate
About This Item
Produits recommandés
Niveau de qualité
Pureté
≥98%
Forme
powder
Pf
171-174 °C (lit.)
Température de stockage
−20°C
Chaîne SMILES
[I-].CCCC(=O)SCC[N+](C)(C)C
InChI
1S/C9H20NOS.HI/c1-5-6-9(11)12-8-7-10(2,3)4;/h5-8H2,1-4H3;1H/q+1;/p-1
Clé InChI
WEQAAFZDJROSBF-UHFFFAOYSA-M
Vous recherchez des produits similaires ? Visite Guide de comparaison des produits
Catégories apparentées
Description générale
Application
- Label-Free and Ultrasensitive Detection of Butyrylcholinesterase: A study demonstrated the use of Mn(II)-based electron spin resonance spectroscopy for the ultrasensitive detection of butyrylcholinesterase, using Butyrylthiocholine iodide as a substrate to quantify enzyme activity in the presence of organophosphorus pesticides, crucial for biochemical assay applications (Tang et al., 2022).
- Novel Nanozyme for Biosensing: Research developed a Co, N co-doped porous carbon-based nanozyme, demonstrating its utility as an oxidase mimic for fluorescence and colorimetric biosensing of butyrylcholinesterase, employing Butyrylthiocholine iodide as a key substrate, relevant in enzyme kinetics analysis (Sun et al., 2022).
- Detection System for Anti-Alzheimer′s Drug Screening: A fluorescent platform was constructed using copper nanoclusters and MnO2 nanosheets for the detection of butyrylcholinesterase activity, utilizing Butyrylthiocholine iodide, which may facilitate the screening of anti-Alzheimer′s drugs and probe cholinergic system interactions (Chen et al., 2022).
- Dual-Channel Detection of Butyrylcholinesterase: A study introduced bifunctional metal-organic frameworks with integrated fluorescence and oxidase activities, developed for dual-channel detection of butyrylcholinesterase using Butyrylthiocholine iodide, enhancing methodologies in biochemical assays (Wang et al., 2022).
Code de la classe de stockage
11 - Combustible Solids
Classe de danger pour l'eau (WGK)
WGK 3
Point d'éclair (°F)
Not applicable
Point d'éclair (°C)
Not applicable
Équipement de protection individuelle
dust mask type N95 (US), Eyeshields, Gloves
Faites votre choix parmi les versions les plus récentes :
Déjà en possession de ce produit ?
Retrouvez la documentation relative aux produits que vous avez récemment achetés dans la Bibliothèque de documents.
Les clients ont également consulté
Notre équipe de scientifiques dispose d'une expérience dans tous les secteurs de la recherche, notamment en sciences de la vie, science des matériaux, synthèse chimique, chromatographie, analyse et dans de nombreux autres domaines..
Contacter notre Service technique