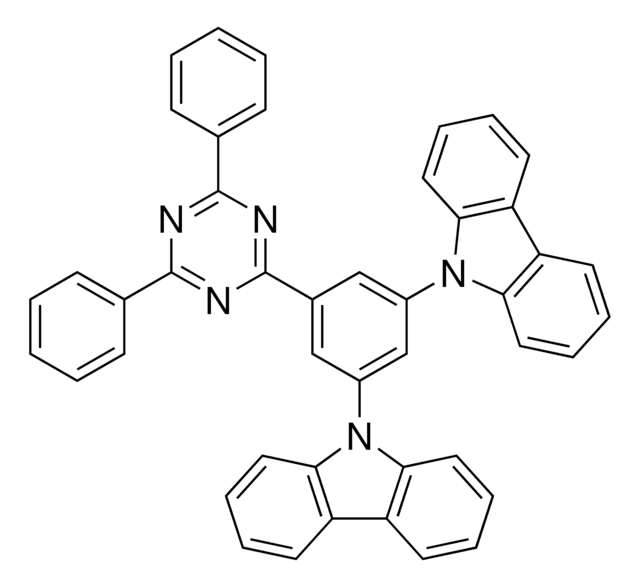
932361
1,3,5-Tri[(3-pyridyl)-phen-3-yl]benzene
≥99% (HPLC)
Synonyme(s) :
TmPyPB
About This Item
Produits recommandés
Qualité
sublimed grade
Niveau de qualité
Description
μe ≈ 1.0 x 10-3 cm2 V−1 s−1
Pureté
≥99% (HPLC)
Perte
0.5% TGA, > 310 °C (weight loss)
Pf
195-200 °C
Température de transition
Tg >310 °C ((0.5% weight loss))
Solubilité
chloroform: soluble
dichloromethane: soluble
Fluorescence
λem 353 nm in dichloromethane (PL)
Énergie orbitale
HOMO 6.75 eV
LUMO 2.75 eV
λ
in dichloromethane
Absorption UV
λ: 254 nm Amax
Chaîne SMILES
C1(C2=CC=CC(C3=CN=CC=C3)=C2)=CC(C4=CC=CC(C5=CN=CC=C5)=C4)=CC(C6=CC=CC(C7=CN=CC=C7)=C6)=C1
InChI
1S/C39H27N3/c1-7-28(34-13-4-16-40-25-34)19-31(10-1)37-22-38(32-11-2-8-29(20-32)35-14-5-17-41-26-35)24-39(23-37)33-12-3-9-30(21-33)36-15-6-18-42-27-36/h1-27H
Clé InChI
CINYXYWQPZSTOT-UHFFFAOYSA-N
Vous recherchez des produits similaires ? Visite Guide de comparaison des produits
Application
Code de la classe de stockage
11 - Combustible Solids
Classe de danger pour l'eau (WGK)
WGK 3
Point d'éclair (°F)
Not applicable
Point d'éclair (°C)
Not applicable
Faites votre choix parmi les versions les plus récentes :
Certificats d'analyse (COA)
Désolés, nous n'avons pas de COA pour ce produit disponible en ligne pour le moment.
Si vous avez besoin d'assistance, veuillez contacter Service Clients
Déjà en possession de ce produit ?
Retrouvez la documentation relative aux produits que vous avez récemment achetés dans la Bibliothèque de documents.
Notre équipe de scientifiques dispose d'une expérience dans tous les secteurs de la recherche, notamment en sciences de la vie, science des matériaux, synthèse chimique, chromatographie, analyse et dans de nombreux autres domaines..
Contacter notre Service technique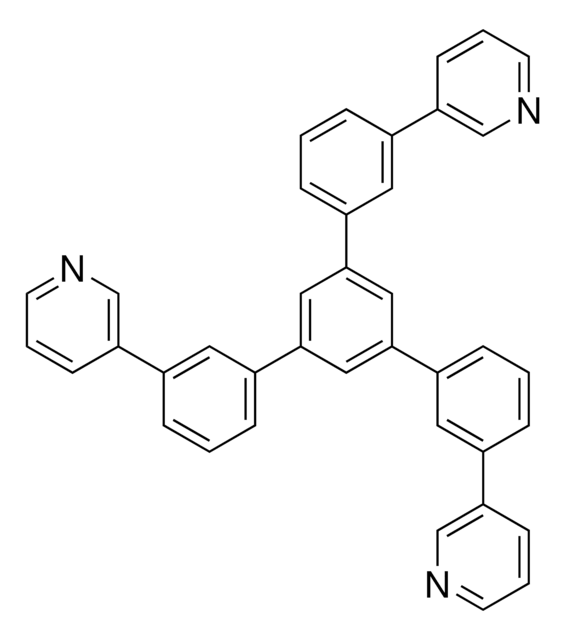





![Di-[4-(N,N-di-p-tolyl-amino)-phenyl]cyclohexane ≥97% (HPLC)](/deepweb/assets/sigmaaldrich/product/structures/111/787/16bde1ce-c76d-46d6-9e1f-9ce09f82d038/640/16bde1ce-c76d-46d6-9e1f-9ce09f82d038.png)