910554
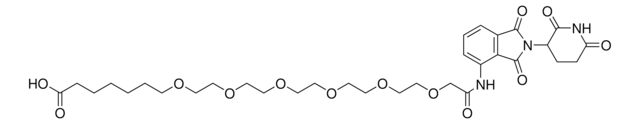
(S,R,S)-AHPC-PEG6-butyl CO2H
≥95%
Synonyme(s) :
(S)-3-((2S,4R)-4-Hydroxy-2-((4-(4-methylthiazol-5-yl)benzyl)carbamoyl)pyrrolidine-1-carbonyl)-2,2-dimethyl-5-oxo-7,10,13,16,19,22-hexaoxa-4-azanonacosan-29-oic acid, (S,R,S)-AHPC-2-2-2-2-2-2-6-Acid, Crosslinker−E3 ligase ligand conjugate, Protein degrader building block for PROTAC® research, Template for synthesis of targeted protein degrader, VH032 conjugate
About This Item
Produits recommandés
ligand
VH032
Pureté
≥95%
Forme
(Liquid or Semi-Solid or Paste or Solid)
Pertinence de la réaction
reactivity: amine reactive
reagent type: ligand-linker conjugate
Groupe fonctionnel
carboxylic acid
Température de stockage
2-8°C
Chaîne SMILES
O=C(N[C@H](C(N1[C@H](C(NCC2=CC=C(C3=C(C)N=CS3)C=C2)=O)C[C@@H](O)C1)=O)C(C)(C)C)COCCOCCOCCOCCOCCOCCCCCCC(O)=O.Cl
Application
Autres remarques
Portal: Building PROTAC® Degraders for Targeted Protein Degradation
Modular PROTAC Design for the Degradation of Oncogenic BCR-ABL
Targeted Protein Degradation by Small Molecules
Small-Molecule PROTACS: New Approaches to Protein Degradation
Targeted Protein Degradation: from Chemical Biology to Drug Discovery
Impact of linker length on the activity of PROTACs
Informations légales
Produit(s) apparenté(s)
Code de la classe de stockage
12 - Non Combustible Liquids
Classe de danger pour l'eau (WGK)
WGK 3
Point d'éclair (°F)
Not applicable
Point d'éclair (°C)
Not applicable
Faites votre choix parmi les versions les plus récentes :
Certificats d'analyse (COA)
Désolés, nous n'avons pas de COA pour ce produit disponible en ligne pour le moment.
Si vous avez besoin d'assistance, veuillez contacter Service Clients
Déjà en possession de ce produit ?
Retrouvez la documentation relative aux produits que vous avez récemment achetés dans la Bibliothèque de documents.
Articles
Protein Degrader Building Blocks are a collection of crosslinker-E3 ligand conjugates with a pendant functional group for covalent linkage to a target ligand.
Protein Degrader Building Blocks are a collection of crosslinker-E3 ligand conjugates with a pendant functional group for covalent linkage to a target ligand.
Protein Degrader Building Blocks are a collection of crosslinker-E3 ligand conjugates with a pendant functional group for covalent linkage to a target ligand.
Protein Degrader Building Blocks are a collection of crosslinker-E3 ligand conjugates with a pendant functional group for covalent linkage to a target ligand.
Notre équipe de scientifiques dispose d'une expérience dans tous les secteurs de la recherche, notamment en sciences de la vie, science des matériaux, synthèse chimique, chromatographie, analyse et dans de nombreux autres domaines..
Contacter notre Service technique