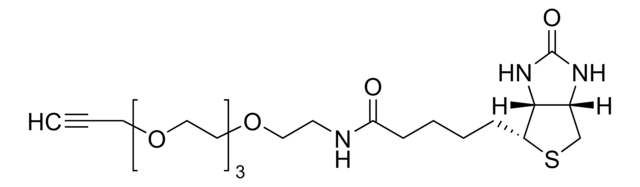
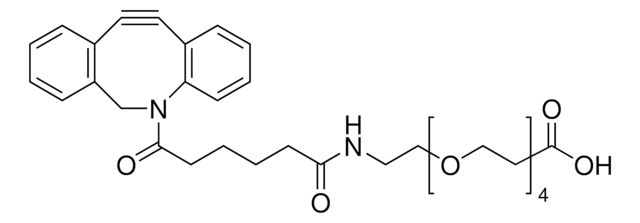
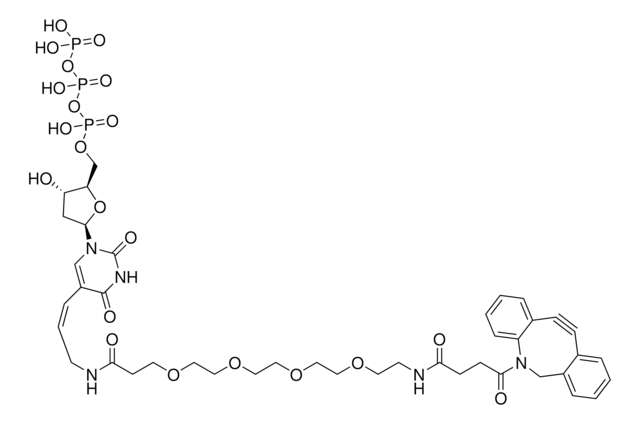
760706
Sulfo-dibenzocyclooctyne-biotin conjugate
for Copper-free Click Chemistry
Synonyme(s) :
Sulfo-DBCO-biotin
About This Item
Produits recommandés
Niveau de qualité
Forme
solid
Capacité de réaction
reaction type: click chemistry
Pertinence de la réaction
reagent type: cross-linking reagent
Température de stockage
−20°C
Chaîne SMILES
CC[NH+](CC)CC.[O-]S(=O)(=O)C(CNC(=O)CCCC[C@@H]1SC[C@@H]2NC(=O)N[C@H]12)C(=O)NCCC(=O)N3Cc4ccccc4C#Cc5ccccc35
InChI
1S/C31H35N5O7S2.C6H15N/c37-27(12-6-5-11-25-29-23(19-44-25)34-31(40)35-29)33-17-26(45(41,42)43)30(39)32-16-15-28(38)36-18-22-9-2-1-7-20(22)13-14-21-8-3-4-10-24(21)36;1-4-7(5-2)6-3/h1-4,7-10,23,25-26,29H,5-6,11-12,15-19H2,(H,32,39)(H,33,37)(H2,34,35,40)(H,41,42,43);4-6H2,1-3H3/t23-,25-,26?,29-;/m0./s1
Clé InChI
RACLEVCKGUBCTH-GESOYTMLSA-N
Description générale
Application
Caractéristiques et avantages
Mention d'avertissement
Warning
Mentions de danger
Conseils de prudence
Classification des risques
Eye Irrit. 2 - Skin Irrit. 2 - STOT SE 3
Organes cibles
Respiratory system
Code de la classe de stockage
11 - Combustible Solids
Classe de danger pour l'eau (WGK)
WGK 3
Point d'éclair (°F)
Not applicable
Point d'éclair (°C)
Not applicable
Faites votre choix parmi les versions les plus récentes :
Déjà en possession de ce produit ?
Retrouvez la documentation relative aux produits que vous avez récemment achetés dans la Bibliothèque de documents.
Les clients ont également consulté
Articles
Copper-free click chemistry is an alternative approach to click chemistry that proceeds at a lower activation barrier and is free of cytotoxic transition metal catalysts.
Copper-free click chemistry is an alternative approach to click chemistry that proceeds at a lower activation barrier and is free of cytotoxic transition metal catalysts.
Copper-free click chemistry is an alternative approach to click chemistry that proceeds at a lower activation barrier and is free of cytotoxic transition metal catalysts.
Copper-free click chemistry is an alternative approach to click chemistry that proceeds at a lower activation barrier and is free of cytotoxic transition metal catalysts.
Notre équipe de scientifiques dispose d'une expérience dans tous les secteurs de la recherche, notamment en sciences de la vie, science des matériaux, synthèse chimique, chromatographie, analyse et dans de nombreux autres domaines..
Contacter notre Service technique






![N-[(1R,8S,9s)-Bicyclo[6.1.0]non-4-yn-9-ylmethyloxycarbonyl]-1,8-diamino-3,6-dioxaoctane for Copper-free Click Chemistry](/deepweb/assets/sigmaaldrich/product/structures/294/853/c5e47d84-5aee-4797-aa24-604f291171cc/640/c5e47d84-5aee-4797-aa24-604f291171cc.png)