543896
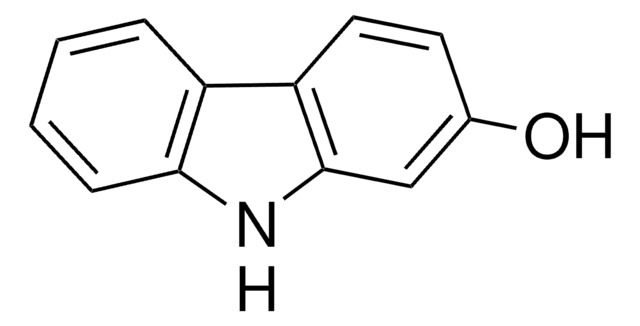
4-Hydroxycarbazole
95%
Synonyme(s) :
9H-Carbazol-4-ol
Se connecterpour consulter vos tarifs contractuels et ceux de votre entreprise/organisme
About This Item
Formule empirique (notation de Hill):
C12H9NO
Numéro CAS:
Poids moléculaire :
183.21
Numéro CE :
Numéro MDL:
Code UNSPSC :
12352100
ID de substance PubChem :
Nomenclature NACRES :
NA.22
Produits recommandés
Niveau de qualité
Pureté
95%
Pf
169-173 °C (lit.)
Chaîne SMILES
Oc1cccc2[nH]c3ccccc3c12
InChI
1S/C12H9NO/c14-11-7-3-6-10-12(11)8-4-1-2-5-9(8)13-10/h1-7,13-14H
Clé InChI
UEOHATPGKDSULR-UHFFFAOYSA-N
Catégories apparentées
Description générale
4-Hydroxycarbazole can be obtained from 1,2,3,4-tetrahydro-4-oxocarbazole via dehydrogenation with freshly prepared Raney nickel.
Application
4-Hydroxycarbazole may be used in the synthesis of the following:
It participates as an electron donor for the preparation of nonlinear optical (NLO) chromophores.
- 4-(2-bromoethoxy)-9H-carbazole
- 4-(3-bromopropoxy)-9H-carbazole
- 4-(4-bromobutoxy)-9H-carbazole
- 4-(5-bromopentyloxy)-9H-carbazole
- 4-(6-bromohexyloxy)-9H-carbazole
It participates as an electron donor for the preparation of nonlinear optical (NLO) chromophores.
Mention d'avertissement
Warning
Mentions de danger
Conseils de prudence
Classification des risques
Eye Irrit. 2 - Skin Irrit. 2 - STOT SE 3
Organes cibles
Respiratory system
Code de la classe de stockage
11 - Combustible Solids
Classe de danger pour l'eau (WGK)
WGK 3
Point d'éclair (°F)
Not applicable
Point d'éclair (°C)
Not applicable
Équipement de protection individuelle
dust mask type N95 (US), Eyeshields, Gloves
Faites votre choix parmi les versions les plus récentes :
Déjà en possession de ce produit ?
Retrouvez la documentation relative aux produits que vous avez récemment achetés dans la Bibliothèque de documents.
Pramod V Chavan et al.
Bioorganic chemistry, 85, 475-486 (2019-02-19)
A series of spirochromenocarbazole tethered 1,2,3-triazoles were synthesized via click chemistry based one-pot, five component reaction between N-propargyl isatins, malononitrile, 4-hydroxycarbazole, aralkyl halides and sodium azide using cellulose supported CuI nanoparticles (Cell-CuI NPs) as the heterogeneous catalyst. Antiproliferative activity of
Kristan H Cleveland et al.
PloS one, 14(5), e0217038-e0217038 (2019-05-21)
Carvedilol is reported to prevent cancers in humans and animal models. However, a molecular mechanism has yet to be established, and the extent to which other β-blockers are chemopreventive remains relatively unknown. A comparative pharmacological approach was utilized with the
Michela Rosini et al.
Journal of medicinal chemistry, 51(15), 4381-4384 (2008-07-09)
Alzheimer's disease (AD) is a multifactorial syndrome with several target proteins contributing to its etiology. To confront AD, an innovative strategy is to design single chemical entities able to simultaneously modulate more than one target. Here, we present compounds that
Synthesis, biological evaluation, and molecular modeling of berberine derivatives as potent acetylcholinesterase inhibitors.
Huang L, et al.
Bioorganic & Medicinal Chemistry, 18(3), 1244-1251 (2010)
E A Dubois et al.
Journal of medicinal chemistry, 39(17), 3256-3262 (1996-08-16)
A new (radio)iodinated, beta-adrenoceptor ligand, (S)-(-)-4-[3-[(1,1-dimethyl-3-iodo-(2E)-propenyl)-amino]-2- hydroxypropoxy]carbazole (CYBL8E, 1), was prepared. 1 is an iodinated analogue of the high-affinity beta-adrenoceptor antagonist carazolol (2). The asymmetric synthesis was achieved in four steps starting from 4-hydroxycarbazole. The iodine-123-labeled form was obtained by
Notre équipe de scientifiques dispose d'une expérience dans tous les secteurs de la recherche, notamment en sciences de la vie, science des matériaux, synthèse chimique, chromatographie, analyse et dans de nombreux autres domaines..
Contacter notre Service technique