447714
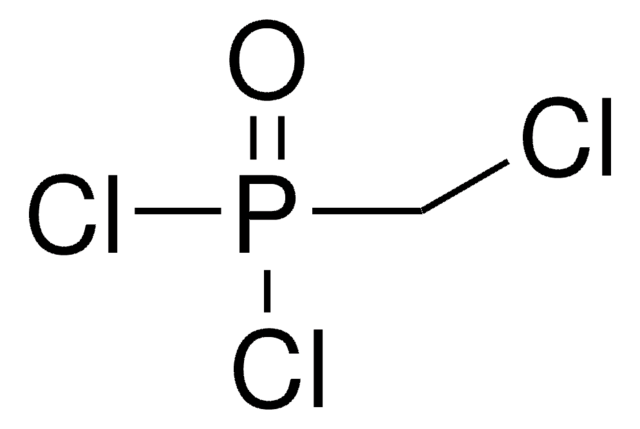
Methylenebis(phosphonic dichloride)
97%
Se connecterpour consulter vos tarifs contractuels et ceux de votre entreprise/organisme
About This Item
Formule linéaire :
CH2[P(O)Cl2]2
Numéro CAS:
Poids moléculaire :
249.78
Numéro MDL:
Code UNSPSC :
12352100
ID de substance PubChem :
Nomenclature NACRES :
NA.22
Produits recommandés
Niveau de qualité
Pureté
97%
Forme
solid
Pf
102-104 °C (lit.)
Groupe fonctionnel
phosphine oxide
Chaîne SMILES
ClP(Cl)(=O)CP(Cl)(Cl)=O
InChI
1S/CH2Cl4O2P2/c2-8(3,6)1-9(4,5)7/h1H2
Clé InChI
VRXYCDTWIOCJBH-UHFFFAOYSA-N
Catégories apparentées
Description générale
Methylenebis(phosphonic dichloride) is an organophosphorus compound that is commonly used in phosphonylation reactions. It is more reactive and the rate of reaction is faster compared to POCl3. This is because the phosphorus center is more electrophilic due to the lack of electron back-donation from the CH2 group.
Application
Methylenebis(phosphonic dichloride) may be used for the following studies:
- Synthesis of mycophenolic methylenebis(phosphonate) derivatives.
- Phosphonylation of nucleosides.
- Preparation of P,P′-partial esters of methylenebisphosphonic acid.
- Synthesis of symmetrical di- and tetra- esters of methylenebisphosphonic acid.
Mention d'avertissement
Danger
Mentions de danger
Classification des risques
Eye Dam. 1 - Skin Corr. 1B
Risques supp
Code de la classe de stockage
8A - Combustible corrosive hazardous materials
Classe de danger pour l'eau (WGK)
WGK 3
Point d'éclair (°F)
Not applicable
Point d'éclair (°C)
Not applicable
Équipement de protection individuelle
Eyeshields, Faceshields, Gloves, type P3 (EN 143) respirator cartridges
Faites votre choix parmi les versions les plus récentes :
Déjà en possession de ce produit ?
Retrouvez la documentation relative aux produits que vous avez récemment achetés dans la Bibliothèque de documents.
Les clients ont également consulté
Krzysztof W Pankiewicz et al.
Journal of medicinal chemistry, 45(3), 703-712 (2002-01-25)
Novel mycophenolic adenine dinucleotide (MAD) analogues have been prepared as potential inhibitors of inosine monophosphate dehydrogenase (IMPDH). MAD analogues resemble nicotinamide adenine dinucleotide binding at the cofactor binding domain of IMPDH; however, they cannot participate in hydride transfer and therefore
Gantla Vidyasagar Reddy et al.
Synthetic communications, 34(2), 331-344 (2004-01-01)
The preparation of partial esters of methylenebisphosphonic acids has been of recent interest due to their potential therapeutic applications. This paper describes a convenient method to prepare symmetrical methylenebis(alkyl hydrogen phosphonates) by the selective cleavage of the corresponding methylenebis(dialkyl phosphonate)
Facile high yielding synthesis of symmetric esters of methylenebisphosphonic acid.
Stepinski DC, et al.
Tetrahedron, 57(41), 8637-8645 (2001)
Aviran Amir et al.
The Journal of organic chemistry, 78(2), 270-277 (2012-12-05)
A new transformation of methylene-bis(phosphonic dichloride) into tetrathiobisphosphonate derivatives is reported. The reaction of methylene-bis(phosphonic dichloride) with 1,2-ethanedithiol in bromoform in the presence of AlCl(3) formed methylene-bis(1,3,2-dithiaphospholane-2-sulfide), which gave rise to O,O'-diester-methylenediphosphonotetrathioate analogues 1a-k upon reaction with phenols and alkyl
Sanjay Bhattarai et al.
Journal of medicinal chemistry, 63(6), 2941-2957 (2020-02-12)
CD73 inhibitors are promising drugs for the (immuno)therapy of cancer. Here, we present the synthesis, structure-activity relationships, and cocrystal structures of novel derivatives of the competitive CD73 inhibitor α,β-methylene-ADP (AOPCP) substituted in the 2-position. Small polar or lipophilic residues increased
Notre équipe de scientifiques dispose d'une expérience dans tous les secteurs de la recherche, notamment en sciences de la vie, science des matériaux, synthèse chimique, chromatographie, analyse et dans de nombreux autres domaines..
Contacter notre Service technique