435171
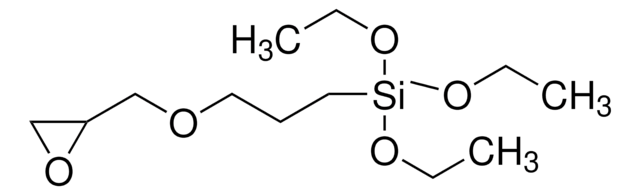
Diethoxy(3-glycidyloxypropyl)methylsilane
97%
Synonyme(s) :
(3-Glycidyloxypropyl)methyldiethoxysilane, [3-(2,3-Epoxypropoxy)propyl]methyldiethoxysilane
About This Item
Produits recommandés
Niveau de qualité
Pureté
97%
Forme
liquid
Indice de réfraction
n20/D 1.431 (lit.)
Point d'ébullition
122-126 °C/5 mmHg (lit.)
Densité
0.978 g/mL at 25 °C (lit.)
Chaîne SMILES
CCO[Si](C)(CCCOCC1CO1)OCC
InChI
1S/C11H24O4Si/c1-4-14-16(3,15-5-2)8-6-7-12-9-11-10-13-11/h11H,4-10H2,1-3H3
Clé InChI
OTARVPUIYXHRRB-UHFFFAOYSA-N
Vous recherchez des produits similaires ? Visite Guide de comparaison des produits
Description générale
Application
Mention d'avertissement
Warning
Mentions de danger
Conseils de prudence
Classification des risques
Eye Irrit. 2 - Skin Irrit. 2 - STOT SE 3
Organes cibles
Respiratory system
Code de la classe de stockage
10 - Combustible liquids
Classe de danger pour l'eau (WGK)
WGK 3
Point d'éclair (°F)
251.6 °F - closed cup
Point d'éclair (°C)
122 °C - closed cup
Équipement de protection individuelle
Eyeshields, Gloves, type ABEK (EN14387) respirator filter
Faites votre choix parmi les versions les plus récentes :
Déjà en possession de ce produit ?
Retrouvez la documentation relative aux produits que vous avez récemment achetés dans la Bibliothèque de documents.
Les clients ont également consulté
Articles
Mesoporous materials, such as aerogels, offer advantages for practical hydrogen storage. They have large surface areas, open porosity, small pore sizes, and the ability to coat the surface with one or more compounds.
Mesoporous materials, such as aerogels, offer advantages for practical hydrogen storage. They have large surface areas, open porosity, small pore sizes, and the ability to coat the surface with one or more compounds.
Mesoporous materials, such as aerogels, offer advantages for practical hydrogen storage. They have large surface areas, open porosity, small pore sizes, and the ability to coat the surface with one or more compounds.
Mesoporous materials, such as aerogels, offer advantages for practical hydrogen storage. They have large surface areas, open porosity, small pore sizes, and the ability to coat the surface with one or more compounds.
Notre équipe de scientifiques dispose d'une expérience dans tous les secteurs de la recherche, notamment en sciences de la vie, science des matériaux, synthèse chimique, chromatographie, analyse et dans de nombreux autres domaines..
Contacter notre Service technique


![[3-(2,3-Epoxypropoxy)-propyl]-trimethoxysilane for synthesis](/deepweb/assets/sigmaaldrich/product/images/156/055/2b7a2b4c-aca4-46e8-a055-64313c26c699/640/2b7a2b4c-aca4-46e8-a055-64313c26c699.jpg)







![3-[Bis(2-hydroxyethyl)amino]propyl-triethoxysilane solution technical, ~65% in ethanol](/deepweb/assets/sigmaaldrich/product/structures/346/789/6478c69e-26fa-46c4-919d-d90c61bc72ee/640/6478c69e-26fa-46c4-919d-d90c61bc72ee.png)

