412805
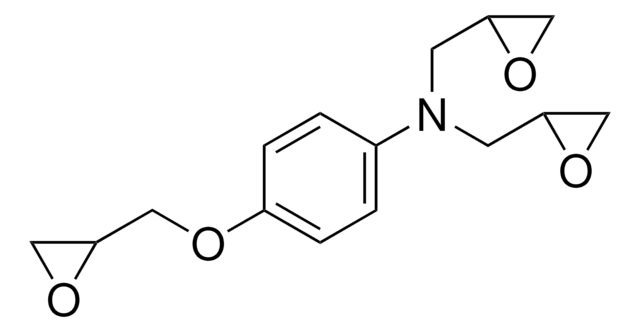
4,4′-Methylenebis(N,N-diglycidylaniline)
Synonyme(s) :
4,4′-Methylenebis[N ,N -bis(oxiranylmethyl)aniline], 4,4′-Methylenedianiline tetraglycidyl ether, N ,N ,N ′,N ′-Tetraglycidyl-4,4′-methylenebisbenzenamine, N ,N ,N ′,N ′-Tetraglycidyl-4,4′-methylenedianiline, Bis[4-(diglycidylamino)phenyl]methane
About This Item
Produits recommandés
Forme
viscous liquid
Niveau de qualité
Indice de réfraction
n20/D 1.601 (lit.)
Densité
1.15 g/mL at 25 °C (lit.)
Chaîne SMILES
C1OC1CN(CC2CO2)c3ccc(Cc4ccc(cc4)N(CC5CO5)CC6CO6)cc3
InChI
1S/C25H30N2O4/c1-5-20(26(10-22-14-28-22)11-23-15-29-23)6-2-18(1)9-19-3-7-21(8-4-19)27(12-24-16-30-24)13-25-17-31-25/h1-8,22-25H,9-17H2
Clé InChI
FAUAZXVRLVIARB-UHFFFAOYSA-N
Description générale
Application
- As a starting material to synthesize UV-curable tetra-functional epoxy acrylate (EA4), which is used as a crosslinker for UV-curable resins.
- In the synthesis of bismaleimide/diallyl bisphenol A (BMI/DBA)–epoxy interpenetrating network resins, which have potential applications in the aerospace and automotive industries because of their high thermal stability and low activation energy.
- As a crosslinking agent in the production of biocompatible materials, such as hydrogels and other tissue engineering scaffolds. Its ability to form strong and stable crosslinks makes it valuable in the creation of medical devices, implants, and drug delivery systems. Additionally, it is utilized in the synthesis of biocompatible polymers and materials with tailored properties for biomedical applications.
- Poly(hexamethylene biguanide) based polymer networks which are applicable as catalysts for the transesterification of vegetable oils.
- Tetra-functional epoxy-acrylate UV curable resins.
Mention d'avertissement
Warning
Mentions de danger
Conseils de prudence
Classification des risques
Aquatic Chronic 2 - Muta. 2 - Skin Sens. 1
Code de la classe de stockage
10 - Combustible liquids
Classe de danger pour l'eau (WGK)
WGK 2
Équipement de protection individuelle
Eyeshields, Faceshields, Gloves, type ABEK (EN14387) respirator filter
Faites votre choix parmi les versions les plus récentes :
Déjà en possession de ce produit ?
Retrouvez la documentation relative aux produits que vous avez récemment achetés dans la Bibliothèque de documents.
Les clients ont également consulté
Notre équipe de scientifiques dispose d'une expérience dans tous les secteurs de la recherche, notamment en sciences de la vie, science des matériaux, synthèse chimique, chromatographie, analyse et dans de nombreux autres domaines..
Contacter notre Service technique