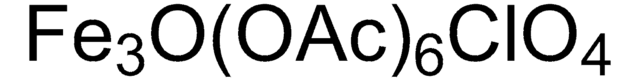399973
Cobalt(II) acetate
99.99% trace metals basis
Synonyme(s) :
Cobalt diacetate, Cobaltous acetate, Cobaltous diacetate
About This Item
Produits recommandés
Niveau de qualité
Pureté
99.99% trace metals basis
Forme
crystals and lumps
solid
Pertinence de la réaction
core: cobalt
Impuretés
≤5% water
Pf
298 °C (dec.) (lit.)
Chaîne SMILES
CC([O-])=O.[Co+2]
InChI
1S/2C2H4O2.Co/c2*1-2(3)4;/h2*1H3,(H,3,4);/q;;+2/p-2
Clé InChI
QAHREYKOYSIQPH-UHFFFAOYSA-L
Vous recherchez des produits similaires ? Visite Guide de comparaison des produits
Description générale
Application
- A precursor to synthesize cobalt titanium oxide catalysts for the oxygen evolution reaction.
- A starting material to prepare polymer stabilized Co nanocatalyst for growing carbon nanofibers.
- A catalyst for direct amination of azoles under mild reaction conditions.
Cobalt(II) acetate can be:
- Used as a cobalt source in the synthesis of Lithium cobalt oxide (LiCoO2), which is a used as a cathode material in lithium-ion batteries.
- Used as a precursor to synthesize cobalt oxide nanoparticles via a simple direct thermal pyrolysis process. Co3O4 nanoparticles further used as a high-capacity anode materials in lithium-ion batteries.
- Used as an additive in the perovskite precursor solution to control the crystal growth and improve the performance of fully screen-printable hole-transport material (HTM)-free mesoporous perovskite solar cells (PSCs).
Mention d'avertissement
Danger
Mentions de danger
Conseils de prudence
Classification des risques
Acute Tox. 4 Oral - Aquatic Acute 1 - Aquatic Chronic 1 - Carc. 1B Inhalation - Eye Irrit. 2 - Muta. 2 - Repr. 1B - Resp. Sens. 1 - Skin Sens. 1
Code de la classe de stockage
6.1D - Non-combustible acute toxic Cat.3 / toxic hazardous materials or hazardous materials causing chronic effects
Classe de danger pour l'eau (WGK)
WGK 3
Équipement de protection individuelle
Eyeshields, Faceshields, Gloves, type P3 (EN 143) respirator cartridges
Faites votre choix parmi les versions les plus récentes :
Déjà en possession de ce produit ?
Retrouvez la documentation relative aux produits que vous avez récemment achetés dans la Bibliothèque de documents.
Les clients ont également consulté
Articles
The diversity of applications and nanostructured materials accessible using ultrasonic spray methods are highlighted in this article.
The diversity of applications and nanostructured materials accessible using ultrasonic spray methods are highlighted in this article.
The diversity of applications and nanostructured materials accessible using ultrasonic spray methods are highlighted in this article.
The diversity of applications and nanostructured materials accessible using ultrasonic spray methods are highlighted in this article.
Notre équipe de scientifiques dispose d'une expérience dans tous les secteurs de la recherche, notamment en sciences de la vie, science des matériaux, synthèse chimique, chromatographie, analyse et dans de nombreux autres domaines..
Contacter notre Service technique