392189
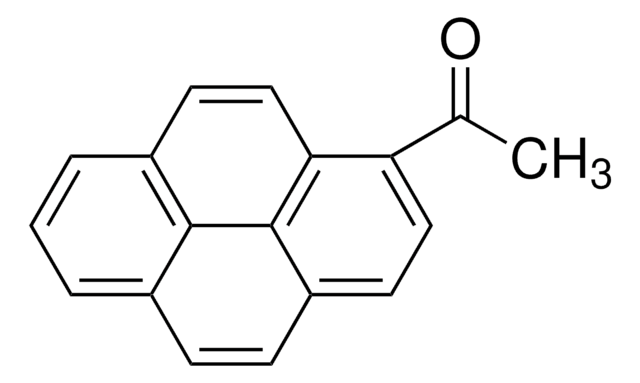
1-Pyreneacetic acid
97%
Synonyme(s) :
(1-Pyrenyl)acetic acid
Se connecterpour consulter vos tarifs contractuels et ceux de votre entreprise/organisme
About This Item
Formule empirique (notation de Hill):
C18H12O2
Numéro CAS:
Poids moléculaire :
260.29
Numéro MDL:
Code UNSPSC :
12352100
ID de substance PubChem :
Nomenclature NACRES :
NA.22
Produits recommandés
Pureté
97%
Forme
solid
Pf
210-212 °C (dec.) (lit.)
Groupe fonctionnel
carboxylic acid
Chaîne SMILES
OC(=O)Cc1ccc2ccc3cccc4ccc1c2c34
InChI
1S/C18H12O2/c19-16(20)10-14-7-6-13-5-4-11-2-1-3-12-8-9-15(14)18(13)17(11)12/h1-9H,10H2,(H,19,20)
Clé InChI
SDJCLYBBPUHKCD-UHFFFAOYSA-N
Description générale
1-Pyreneacetic acid is a negatively charged pyrene derivative. It has been proposed as titrating reagent for the standardization titration of Grignard reagents and n-butyl lithium (n-BuLi).
Application
1-Pyreneacetic acid is suitable for use in the following studies:
- Synthesis of N-(2-(methylthio)ethyl)-2-(pyren-1-yl)acetamide, a pyrene amide based Pd2+ probe.
- Synthesis of pyrene-modified β-cyclodextrin.
- To functionalize single walled carbon nanotube field effect transistors (CNT FETs).
- As an agent for characterizing grafting degrees and reactivity of the ester functionalized polypropylenes.
- Synthesis sawhorse-type diruthenium tetracarbonyl complexes.
- Synthesis of (±)-2-(1-pyrenyl)propionic acid, a chiral carboxylic acid.
- Reversible noncovalent functionalization of single walled carbon nanotubes (SWNTs).
- Preparation of peptide nucleic acid (PNA) probes.
- As an internal reference compound in the assessment of solid phase reaction by HPLC-UV.
Code de la classe de stockage
11 - Combustible Solids
Classe de danger pour l'eau (WGK)
WGK 3
Point d'éclair (°F)
Not applicable
Point d'éclair (°C)
Not applicable
Équipement de protection individuelle
Eyeshields, Gloves, type N95 (US)
Faites votre choix parmi les versions les plus récentes :
Déjà en possession de ce produit ?
Retrouvez la documentation relative aux produits que vous avez récemment achetés dans la Bibliothèque de documents.
Les clients ont également consulté
Fushen Lu et al.
Langmuir : the ACS journal of surfaces and colloids, 26(10), 7561-7564 (2010-01-06)
An effective purification method for single-walled carbon nanotubes (SWNTs) based on a combination of oxidative acid treatment and reversible noncovalent functionalization with 1-pyreneacetic acid is reported. The functionalization was selective toward the nanotubes, allowing a nearly complete removal of residual
Ali Khalil et al.
Polymers, 12(8) (2020-08-06)
Hydrophobic and amphiphilic derivatives of the biocompatible and biodegradable poly(dimethylmalic acid) (PdiMeMLA), varying by the nature of the lateral chains and the length of each block, respectively, have been synthesized by anionic ring-opening polymerization (aROP) of the corresponding monomers using
Sawhorse-type diruthenium tetracarbonyl complexes derived from pyrenyl-carboxylic acids.
Johnpeter JP and Therrien B.
Inorgorganica Chimica Acta, 405, 437-443 (2013)
Jan Spengler et al.
ACS combinatorial science, 15(5), 229-234 (2013-03-26)
Here we evaluated the use of internal reference compounds for the rapid assessment of reactions performed in solid-phase. An internal reference compound (commercially available) was bound to the resin, together with the substrate, and cleaved with the products after completion
Gabriela Ramos Chagas et al.
Chemphyschem : a European journal of chemical physics and physical chemistry, 18(23), 3429-3436 (2017-09-01)
A smart stimuli-responsive surface was fabricated by the electro-copolymerization of pyrene monomers followed by base and acid treatment. Copolymers of pyrenes bearing fluorinated chains (Py-nF
Notre équipe de scientifiques dispose d'une expérience dans tous les secteurs de la recherche, notamment en sciences de la vie, science des matériaux, synthèse chimique, chromatographie, analyse et dans de nombreux autres domaines..
Contacter notre Service technique