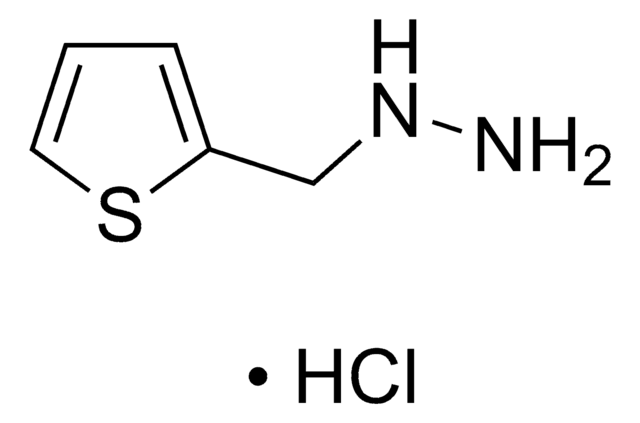
284335
1,3-Dinitropyrene
99%
About This Item
Produits recommandés
Pureté
99%
Forme
solid
Solubilité
DMSO: soluble 2 mg/mL, clear, yellow to orange
Groupe fonctionnel
nitro
Chaîne SMILES
[O-][N+](=O)c1cc([N+]([O-])=O)c2ccc3cccc4ccc1c2c34
InChI
1S/C16H8N2O4/c19-17(20)13-8-14(18(21)22)12-7-5-10-3-1-2-9-4-6-11(13)16(12)15(9)10/h1-8H
Clé InChI
KTNUVDBUEAQUON-UHFFFAOYSA-N
Catégories apparentées
Description générale
Application
- modification of the umu-assay (ISO 13829) to assess the cytotoxic potential of toxins
- in vitro synthesis of 1,N6-etheno-2′-deoxyadenosine and 1,N2-etheno-2′-deoxyguanosine
Mention d'avertissement
Warning
Mentions de danger
Conseils de prudence
Classification des risques
Acute Tox. 4 Dermal - Acute Tox. 4 Inhalation - Acute Tox. 4 Oral
Code de la classe de stockage
11 - Combustible Solids
Classe de danger pour l'eau (WGK)
WGK 3
Point d'éclair (°F)
Not applicable
Point d'éclair (°C)
Not applicable
Équipement de protection individuelle
dust mask type N95 (US), Eyeshields, Gloves
Faites votre choix parmi les versions les plus récentes :
Déjà en possession de ce produit ?
Retrouvez la documentation relative aux produits que vous avez récemment achetés dans la Bibliothèque de documents.
Notre équipe de scientifiques dispose d'une expérience dans tous les secteurs de la recherche, notamment en sciences de la vie, science des matériaux, synthèse chimique, chromatographie, analyse et dans de nombreux autres domaines..
Contacter notre Service technique






![1-(6-Methoxybenzo[d] thiazol-2-yl)hydrazine AldrichCPR](/deepweb/assets/sigmaaldrich/product/structures/210/241/0c5be390-b73a-436d-82c7-c51156617e66/640/0c5be390-b73a-436d-82c7-c51156617e66.png)