128635
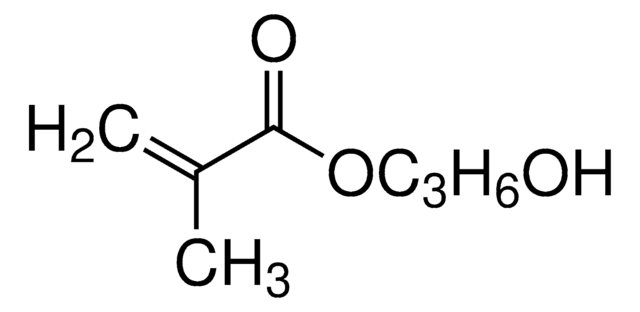
2-Hydroxyethyl methacrylate
contains ≤250 ppm monomethyl ether hydroquinone as inhibitor, 97%
Synonyme(s) :
1,2-Ethanediol mono(2-methylpropenoate), Glycol methacrylate, HEMA
About This Item
Produits recommandés
Densité de vapeur
5 (vs air)
Niveau de qualité
Pression de vapeur
0.01 mmHg ( 25 °C)
Pureté
97%
Forme
liquid
Contient
≤250 ppm monomethyl ether hydroquinone as inhibitor
Indice de réfraction
n20/D 1.453 (lit.)
Point d'ébullition
67 °C/3.5 mmHg (lit.)
Densité
1.073 g/mL at 25 °C (lit.)
Température de stockage
2-8°C
Chaîne SMILES
CC(=C)C(=O)OCCO
InChI
1S/C6H10O3/c1-5(2)6(8)9-4-3-7/h7H,1,3-4H2,2H3
Clé InChI
WOBHKFSMXKNTIM-UHFFFAOYSA-N
Vous recherchez des produits similaires ? Visite Guide de comparaison des produits
Catégories apparentées
Description générale
Application
Mention d'avertissement
Warning
Mentions de danger
Conseils de prudence
Classification des risques
Eye Irrit. 2 - Skin Irrit. 2 - Skin Sens. 1
Code de la classe de stockage
10 - Combustible liquids
Classe de danger pour l'eau (WGK)
WGK 1
Point d'éclair (°F)
222.8 °F - closed cup
Point d'éclair (°C)
106 °C - closed cup
Faites votre choix parmi les versions les plus récentes :
Déjà en possession de ce produit ?
Retrouvez la documentation relative aux produits que vous avez récemment achetés dans la Bibliothèque de documents.
Les clients ont également consulté
Articles
Monomers for ophthalmic use aim for purity, reliability, and comfort, driving innovation for affordable contact lenses.
Monomers for ophthalmic use aim for purity, reliability, and comfort, driving innovation for affordable contact lenses.
Monomers for ophthalmic use aim for purity, reliability, and comfort, driving innovation for affordable contact lenses.
Monomers for ophthalmic use aim for purity, reliability, and comfort, driving innovation for affordable contact lenses.
Notre équipe de scientifiques dispose d'une expérience dans tous les secteurs de la recherche, notamment en sciences de la vie, science des matériaux, synthèse chimique, chromatographie, analyse et dans de nombreux autres domaines..
Contacter notre Service technique