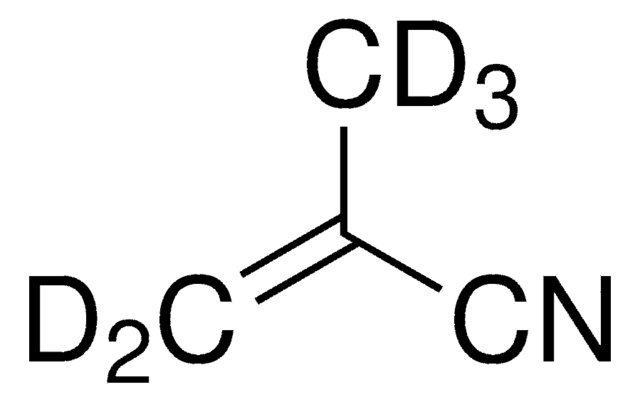
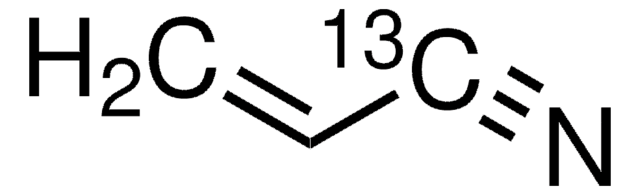
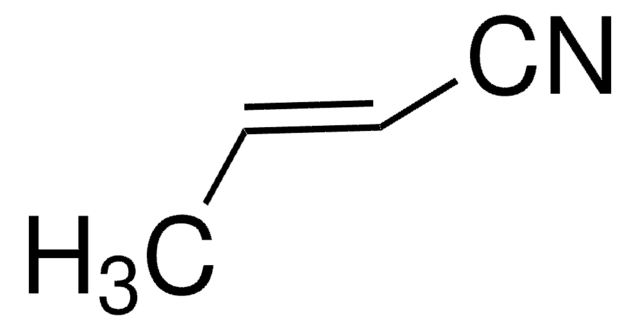
110213
Acrylonitrile
≥99%, contains 35-45 ppm monomethyl ether hydroquinone as inhibitor
Synonyme(s) :
Vinyl cyanide
About This Item
Produits recommandés
Densité de vapeur
1.83 (vs air)
Niveau de qualité
Pression de vapeur
86 mmHg ( 20 °C)
Pureté
≥99%
Température d'inflammation spontanée
897 °F
Contient
35-45 ppm monomethyl ether hydroquinone as inhibitor
Limite d'explosivité
17 %
Indice de réfraction
n20/D 1.391 (lit.)
Point d'ébullition
77 °C (lit.)
Pf
−83 °C (lit.)
Chaîne SMILES
C=CC#N
InChI
1S/C3H3N/c1-2-3-4/h2H,1H2
Clé InChI
NLHHRLWOUZZQLW-UHFFFAOYSA-N
Vous recherchez des produits similaires ? Visite Guide de comparaison des produits
Description générale
Application
- In the preparation of the 3D-printed polymer material, Acrylonitrile Butadiene Styrene (ABS) which is a commonly used engineering thermoplastic known for its high strength, durability, and heat resistance. It serves as a suitable substrate for a wide range of applications, including in the medical field, compatible manufacturing processes, injection molding, blow molding, and extrusion.
- In the copolymerization with lignosulfonate to develop a carbon fiber precursor. This copolymer can serve as a precursor material that undergoes further thermal treatment to produce carbon fibers.
- To synthesize acrylamide grafted acrylonitrile copolymer membranes, which serve as a support matrix for the immobilization of cellulase enzymes.
Actions biochimiques/physiologiques
Mention d'avertissement
Danger
Mentions de danger
Conseils de prudence
Classification des risques
Acute Tox. 3 Dermal - Acute Tox. 3 Inhalation - Acute Tox. 3 Oral - Aquatic Chronic 2 - Carc. 1B - Eye Dam. 1 - Flam. Liq. 2 - Skin Irrit. 2 - Skin Sens. 1B - STOT SE 3
Organes cibles
Respiratory system
Code de la classe de stockage
3 - Flammable liquids
Classe de danger pour l'eau (WGK)
WGK 3
Point d'éclair (°F)
23.0 °F - closed cup
Point d'éclair (°C)
-5 °C - closed cup
Équipement de protection individuelle
Faceshields, Gloves, Goggles, type ABEK (EN14387) respirator filter
Faites votre choix parmi les versions les plus récentes :
Déjà en possession de ce produit ?
Retrouvez la documentation relative aux produits que vous avez récemment achetés dans la Bibliothèque de documents.
Les clients ont également consulté
Notre équipe de scientifiques dispose d'une expérience dans tous les secteurs de la recherche, notamment en sciences de la vie, science des matériaux, synthèse chimique, chromatographie, analyse et dans de nombreux autres domaines..
Contacter notre Service technique