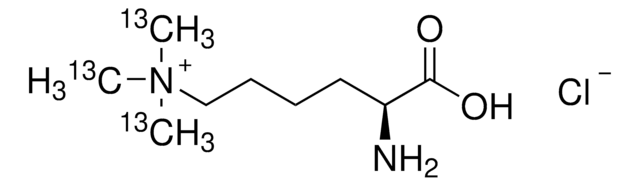
T1660
Nε,Nε,Nε-Trimethyllysine hydrochloride
≥97% (TLC)
Synonym(s):
1-Pentanaminium, 5-amino-5-carboxy-N,N,N-trimethyl-, chloride (1:1), (5S)-
Sign Into View Organizational & Contract Pricing
All Photos(1)
About This Item
Empirical Formula (Hill Notation):
C9H20N2O2 · HCl
CAS Number:
Molecular Weight:
224.73
MDL number:
UNSPSC Code:
12352202
PubChem Substance ID:
NACRES:
NA.26
Recommended Products
Assay
≥97% (TLC)
form
powder
contains
salts and water as balance
composition
Amino acid content, ~75%
technique(s)
LC/MS: suitable
color
white
storage temp.
−20°C
SMILES string
[Cl-].C[N+](C)(C)CCCC[C@H](N)C(O)=O
InChI
1S/C9H20N2O2.ClH/c1-11(2,3)7-5-4-6-8(10)9(12)13;/h8H,4-7,10H2,1-3H3;1H/t8-;/m0./s1
InChI key
ZKIJKCHCMFGMCM-QRPNPIFTSA-N
Related Categories
Storage Class Code
11 - Combustible Solids
WGK
WGK 3
Flash Point(F)
Not applicable
Flash Point(C)
Not applicable
Personal Protective Equipment
dust mask type N95 (US), Eyeshields, Gloves
Choose from one of the most recent versions:
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Customers Also Viewed
C J Rebouche et al.
The Journal of nutrition, 116(5), 751-759 (1986-05-01)
Rates of carnitine biosynthesis in mammals depend on the availability of substrates and the activity of enzymes subserving the pathway. This study was undertaken to test the hypothesis that the availability of epsilon-N-trimethyllysine is rate-limiting for synthesis of carnitine in
Karin Strijbis et al.
IUBMB life, 62(5), 357-362 (2010-03-23)
The water-soluble zwitterion carnitine is an essential metabolite in eukaryotes required for fatty acid oxidation as it functions as a carrier during transfer of activated acyl and acetyl groups across intracellular membranes. Most eukaryotes are able to synthesize carnitine endogenously
Frank Hirche et al.
Journal of chromatography. B, Analytical technologies in the biomedical and life sciences, 877(22), 2158-2162 (2009-06-16)
For the investigation of the metabolism and biosynthesis of carnitine, sensitive determination of carnitine and its metabolic precursors, trimethyllysine and gamma-butyrobetaine, is required. We present here a new simplified method for the analysis of carnitine, its acetyl- and propyl esters
Naomi van Vlies et al.
Analytical biochemistry, 354(1), 132-139 (2006-05-19)
Although the mouse frequently is used to study metabolism and deficiencies therein, little is known about carnitine biosynthesis in this animal. To this point, only laborious procedures have been described to measure the activity of carnitine biosynthesis enzymes using subcellular
Lynnette M A Dirk et al.
Biochemistry, 46(12), 3905-3915 (2007-03-07)
Processive versus distributive methyl group transfer was assessed for pea Rubisco large subunit methyltransferase, a SET domain protein lysine methyltransferase catalyzing the formation of trimethyllysine-14 in the large subunit of Rubisco. Catalytically competent complexes between an immobilized form of des(methyl)
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service