03820585
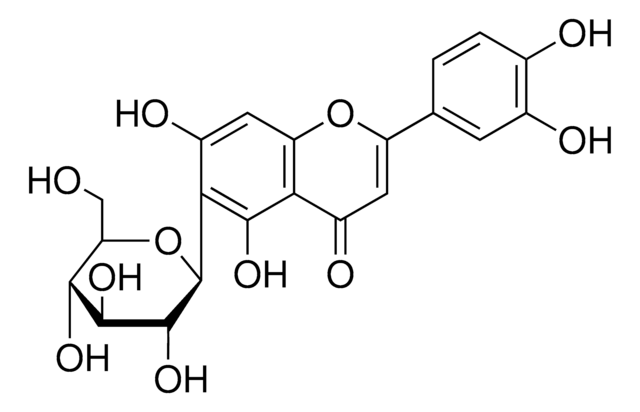
Isoorientin
primary reference standard
Synonym(s):
Homoorientin, Luteolin 6-C-β-D-glucoside, Luteolin 6-C-glucoside
About This Item
Recommended Products
grade
primary reference standard
shelf life
limited shelf life, expiry date on the label
manufacturer/tradename
HWI
application(s)
food and beverages
SMILES string
OC[C@H]1O[C@H]([C@H](O)[C@@H](O)[C@@H]1O)c2c(O)cc3OC(=CC(=O)c3c2O)c4ccc(O)c(O)c4
InChI
1S/C21H20O11/c22-6-14-17(27)19(29)20(30)21(32-14)16-11(26)5-13-15(18(16)28)10(25)4-12(31-13)7-1-2-8(23)9(24)3-7/h1-5,14,17,19-24,26-30H,6H2/t14-,17-,19+,20-,21+/m1/s1
InChI key
ODBRNZZJSYPIDI-VJXVFPJBSA-N
Looking for similar products? Visit Product Comparison Guide
General description
Exact content by quantitative NMR can be found on the certificate.
Application
Biochem/physiol Actions
Storage Class Code
11 - Combustible Solids
WGK
WGK 3
Flash Point(F)
Not applicable
Flash Point(C)
Not applicable
Choose from one of the most recent versions:
Certificates of Analysis (COA)
Don't see the Right Version?
If you require a particular version, you can look up a specific certificate by the Lot or Batch number.
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Protocols
HPTLC fingerprinting enables rapid identification of passion flower with related reference materials.
HPTLC fingerprinting enables rapid identification of passion flower with related reference materials.
HPTLC fingerprinting enables rapid identification of passion flower with related reference materials.
HPTLC fingerprinting enables rapid identification of passion flower with related reference materials.
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service