About This Item
Recommended Products
biological source
synthetic
Quality Level
grade
FG
Halal
Kosher
reg. compliance
EU Regulation 1334/2008 & 178/2002
FDA 21 CFR 117
FDA 21 CFR 172.515
Assay
≥96%
mp
39.0-45.0 °C (lit.)
application(s)
flavors and fragrances
Documentation
see Safety & Documentation for available documents
food allergen
no known allergens
Organoleptic
earthy; camphoraceous; pine
SMILES string
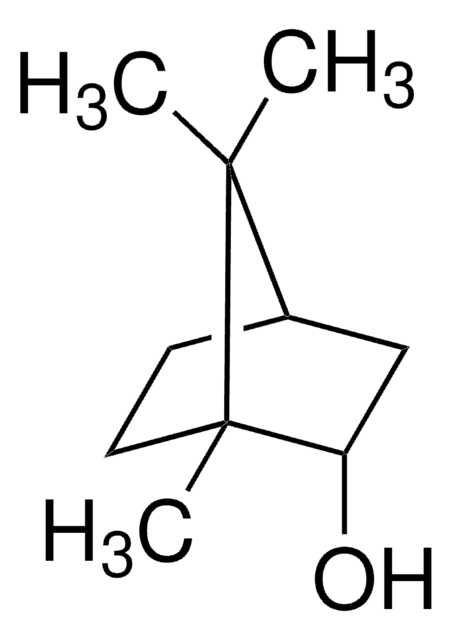
[H][C@]12CC[C@](C)(C1)[C@@H](O)C2(C)C
InChI
1S/C10H18O/c1-9(2)7-4-5-10(3,6-7)8(9)11/h7-8,11H,4-6H2,1-3H3/t7-,8-,10+/m0/s1
InChI key
IAIHUHQCLTYTSF-OYNCUSHFSA-N
Looking for similar products? Visit Product Comparison Guide
Application
- Enhancing the NMR signals of plant oil components using hyperpolarisation relayed via proton exchange.: This study explores the enhancement of NMR signals of plant oil components, including fenchyl alcohol, using hyperpolarization techniques, which significantly improve the detection sensitivity in NMR spectroscopy (Alshehri et al., 2023).
- Update to RIFM fragrance ingredient safety assessment, fenchyl alcohol, CAS Registry Number 1632-73-1.: This paper provides an updated safety assessment of fenchyl alcohol as a fragrance ingredient, focusing on its toxicological profile and safe usage levels (Api et al., 2022).
- Olfactory Impact of Terpene Alcohol on Terpenes Aroma Expression in Chrysanthemum Essential Oils.: The research investigates how fenchyl alcohol and other terpene alcohols influence the aroma profile of chrysanthemum essential oils, highlighting its significant role in enhancing aromatic properties (Niu et al., 2018).
- Antibiofilm and Antihyphal Activities of Cedar Leaf Essential Oil, Camphor, and Fenchone Derivatives against Candida albicans.: This study demonstrates the antibiofilm and antihyphal activities of fenchyl alcohol derivatives against Candida albicans, suggesting potential applications in antifungal treatments (Manoharan et al., 2017).
- Comparative Study on Volatile Compounds of Alpinia japonica and Elettaria cardamomum.: The paper compares the volatile compounds of Alpinia japonica and Elettaria cardamomum, identifying fenchyl alcohol as a key constituent, and discusses its implications for flavor and fragrance applications (Asakawa et al., 2017).
Signal Word
Warning
Hazard Statements
Precautionary Statements
Hazard Classifications
Eye Irrit. 2 - Skin Irrit. 2 - STOT SE 3
Target Organs
Respiratory system
Storage Class Code
11 - Combustible Solids
WGK
WGK 2
Flash Point(F)
165.2 °F - closed cup
Flash Point(C)
74 °C - closed cup
Certificates of Analysis (COA)
Search for Certificates of Analysis (COA) by entering the products Lot/Batch Number. Lot and Batch Numbers can be found on a product’s label following the words ‘Lot’ or ‘Batch’.
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service