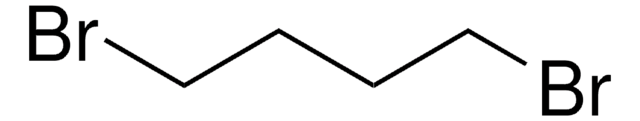
382205
2-Bromoethyl ether
technical grade, 90%
Synonym(s):
Bis(2-bromoethyl) ether
Sign Into View Organizational & Contract Pricing
All Photos(1)
About This Item
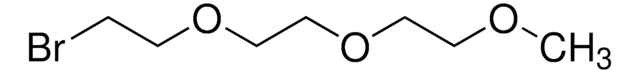
Linear Formula:
(BrCH2CH2)2O
CAS Number:
Molecular Weight:
231.91
EC Number:
MDL number:
UNSPSC Code:
12352100
PubChem Substance ID:
NACRES:
NA.22
Recommended Products
grade
technical grade
Assay
90%
form
liquid
bp
92-93 °C/12 mmHg (lit.)
density
1.845 g/mL at 25 °C (lit.)
storage temp.
2-8°C
SMILES string
BrCCOCCBr
InChI
1S/C4H8Br2O/c5-1-3-7-4-2-6/h1-4H2
InChI key
FOZVXADQAHVUSV-UHFFFAOYSA-N
Related Categories
General description
2-Bromoethyl ether (2,2′-Dibromodiethyl ether) is a halogen containing ether. 2,2′-Dibromodiethyl ether has been prepared by reacting dioxane with anhydrous, bromine free hydrogen bromide.
Application
2-Bromoethyl ether (2,2′-Dibromodiethyl ether) may be used in the preparation of 1,4,7-trioxa-10-19-dithia-13,16-diaza-12,17-dioxo-8,9,14,15,20,21-tribenzoheneicosane.
2-Bromoethyl ether may be used in the synthesis of macroheterocycles including redox-active tetrathiafulvalene linked systems and polyoxaza cryptands.
Signal Word
Danger
Hazard Statements
Precautionary Statements
Hazard Classifications
Eye Dam. 1 - Skin Irrit. 2 - STOT SE 3
Storage Class Code
10 - Combustible liquids
WGK
WGK 3
Flash Point(F)
185.0 °F
Flash Point(C)
85 °C
Personal Protective Equipment
dust mask type N95 (US), Eyeshields, Gloves
Certificates of Analysis (COA)
Search for Certificates of Analysis (COA) by entering the products Lot/Batch Number. Lot and Batch Numbers can be found on a product’s label following the words ‘Lot’ or ‘Batch’.
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Customers Also Viewed
J. Chem. Soc. Perkin Trans. II, 1213-1213 (1989)
Journal of the Chemical Society. Chemical Communications, 1550-1550 (1992)
The reaction of dioxane with hydrogen bromide.
Cleave ABV and Blake RI.
Canadian Journal of Chemistry, 29(9), 785-789 (1951)
Ping-Yu Wu et al.
The Journal of organic chemistry, 71(2), 833-835 (2006-01-18)
[reaction: see text] A mild asymmetric arylation of aromatic aldehydes catalyzed by gamma-amino thiol 5 gave the corresponding diarylmethanols with 95 to >99.5% ee.
New approach to the synthesis of dibenzodithia-and benzothiaazacrown ethers via the aromatization of 2-alkylthio (arylthio) cyclohexanes during bromination.
Kudryatsev KV and Samofin VV.
Chemistry of Heterocyclic Compounds, 33(1), 106-111 (1997)
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service


![Bis[2-(2-chloroethoxy)ethyl] ether ≥99.0% (T)](/deepweb/assets/sigmaaldrich/product/structures/333/320/46ff3398-7a62-42b5-b9bc-0a3d0cb0429c/640/46ff3398-7a62-42b5-b9bc-0a3d0cb0429c.png)