All Photos(2)
About This Item
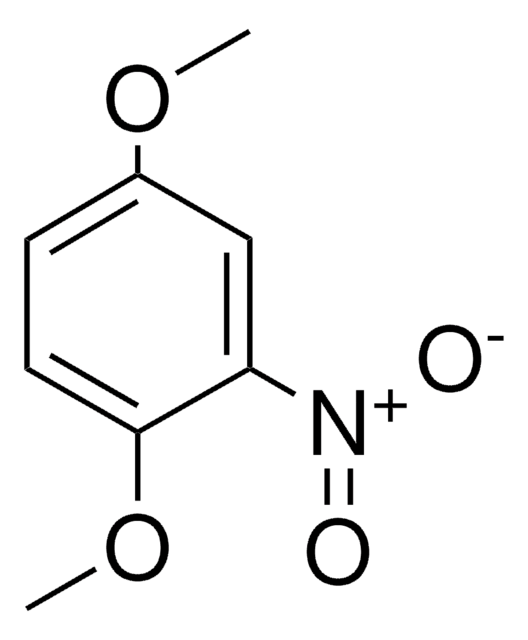
Linear Formula:
CH3C6H3(NO2)OCH3
CAS Number:
Molecular Weight:
167.16
EC Number:
MDL number:
UNSPSC Code:
12352100
PubChem Substance ID:
NACRES:
NA.22
Recommended Products
Quality Level
Assay
99%
form
liquid
refractive index
n20/D 1.557 (lit.)
bp
154 °C/14 mmHg (lit.)
mp
8-9 °C (lit.)
density
1.205 g/mL at 25 °C (lit.)
SMILES string
COc1ccc(C)cc1[N+]([O-])=O
InChI
1S/C8H9NO3/c1-6-3-4-8(12-2)7(5-6)9(10)11/h3-5H,1-2H3
InChI key
LGNMURXRPLMVJI-UHFFFAOYSA-N
Related Categories
General description
Nucleophilic substitution reactions of 4-methyl-2-nitroanisole in neat cyclohexylamine and piperidine have been reported.
Application
4-Methyl-2-nitroanisole may be used in the synthesis of 1-dibromomethyl-4-methoxy-2-nitrobenzene.
Signal Word
Warning
Hazard Statements
Precautionary Statements
Hazard Classifications
Acute Tox. 4 Oral
Storage Class Code
10 - Combustible liquids
WGK
WGK 3
Flash Point(F)
235.4 °F - closed cup
Flash Point(C)
113 °C - closed cup
Personal Protective Equipment
dust mask type N95 (US), Eyeshields, Gloves
Certificates of Analysis (COA)
Search for Certificates of Analysis (COA) by entering the products Lot/Batch Number. Lot and Batch Numbers can be found on a product’s label following the words ‘Lot’ or ‘Batch’.
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Hoong-Kun Fun et al.
Acta crystallographica. Section E, Structure reports online, 65(Pt 9), o2193-o2194 (2009-01-01)
The asymmetric unit of the title compound, C(8)H(7)Br(2)NO(3), comprises two crystallographically independent mol-ecules (A and B). The nitro groups are twisted from the attached benzene rings, making dihedral angles of 39.26 (9) and 35.90 (9)° in mol-ecules A and B, respectively. In
Theoretical calculations of chemical interactions. Part 4. Aromatic nucleophilic substitutions and SN 2 reactions of 4-and 6-substituted 2-nitroanisoles.
Nudelman NS and Palleros DR.
J. Chem. Soc. Perkin Trans. II, 6, 805-809 (1985)
Yusuke Yamamoto et al.
The Journal of toxicological sciences, 44(9), 585-600 (2019-09-03)
Amino acid derivative reactivity assay (ADRA) has previously been developed as an alternative method to direct peptide reactivity assay (DPRA) to evaluate key event 1 in skin sensitization mechanisms. However, when using alternative methods for skin sensitization, integrated approaches to
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service