All Photos(1)
About This Item
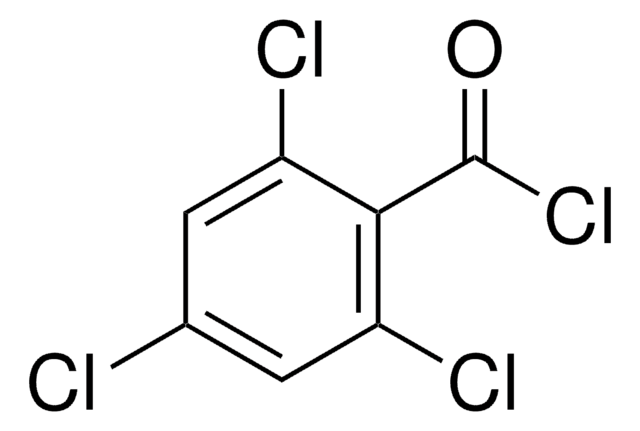
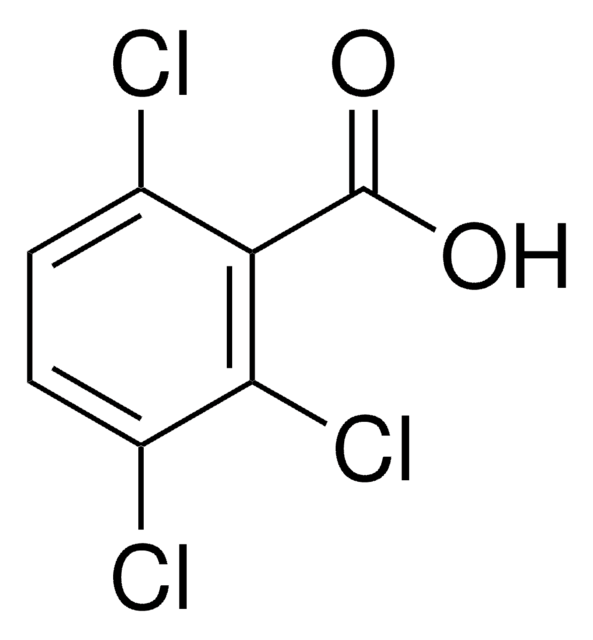
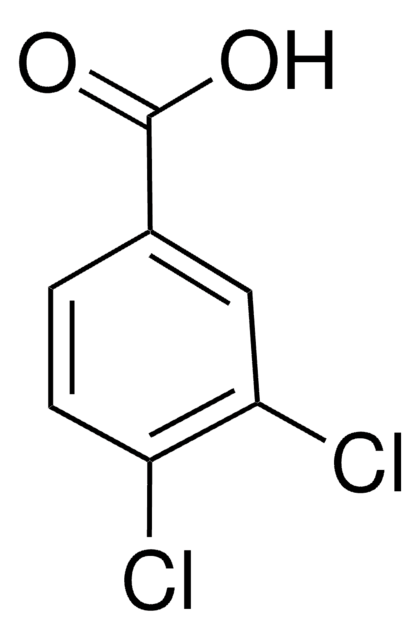
Linear Formula:
Cl3C6H2CO2H
CAS Number:
Molecular Weight:
225.46
Beilstein:
2097064
MDL number:
UNSPSC Code:
12352100
PubChem Substance ID:
NACRES:
NA.22
Recommended Products
Quality Level
Assay
97%
form
solid
mp
166-167 °C (lit.)
functional group
carboxylic acid
chloro
SMILES string
OC(=O)c1cc(Cl)cc(Cl)c1Cl
InChI
1S/C7H3Cl3O2/c8-3-1-4(7(11)12)6(10)5(9)2-3/h1-2H,(H,11,12)
InChI key
CGFDSIZRJWMQPP-UHFFFAOYSA-N
Looking for similar products? Visit Product Comparison Guide
Related Categories
Signal Word
Warning
Hazard Statements
Precautionary Statements
Hazard Classifications
Eye Irrit. 2 - Skin Irrit. 2 - STOT SE 3
Target Organs
Respiratory system
Storage Class Code
11 - Combustible Solids
WGK
WGK 3
Flash Point(F)
Not applicable
Flash Point(C)
Not applicable
Personal Protective Equipment
dust mask type N95 (US), Eyeshields, Gloves
Choose from one of the most recent versions:
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Effects of temperature on biological degradation of phenols, benzoates and phthalates under methanogenic conditions.
Leven L and Schnurer A.
International Biodeterioration & Biodegradation, 55(2), 153-160 (2005)
Rearrangement of Halotropones. Chloride Exchange in Tribromotropolone.
Doering WE and Knox LH.
Journal of the American Chemical Society, 74(22), 5683-5687 (1952)
Meng Qi et al.
Analytical and bioanalytical chemistry, 412(25), 6995-7006 (2020-08-02)
Dicamba herbicide is increasingly used in the world, in particular' with the widespread cultivation of genetically modified dicamba-resistant crops. However, the drift problem in the field has caused phytotoxicity against naive, sensitive crops, raising legal concerns. Thus, it is particularly
Prakash Karegoudar et al.
European journal of medicinal chemistry, 43(4), 808-815 (2007-09-07)
The reaction of 2,3,5-trichlorobenzoic acid hydrazide with carbon disulfide and potassium hydroxide followed by treatment with hydrazine hydrate afforded 3-(2,3,5-trichlorophenyl)-4-amino-1,2,4-triazole-5-thione (6). Alternatively, this triazole was also synthesized by fusing 2,3,5-trichlorobenzoic acid with thiocarbohydrazide. Condensation of (6) with various aromatic carboxylic
Emeline L Maillet et al.
Journal of medicinal chemistry, 52(21), 6931-6935 (2009-10-13)
We show that phenoxyauxin herbicides and lipid-lowering fibrates inhibit human but not rodent T1R3. T1R3 as a coreceptor in taste cells responds to sweet compounds and amino acids; in endocrine cells of gut and pancreas T1R3 contributes to glucose sensing.
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service